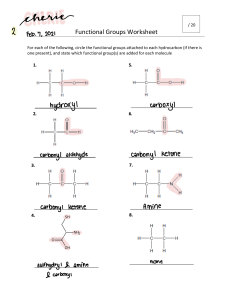
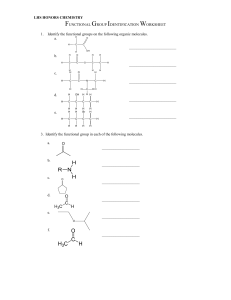
Name: ________________________ Class: ___________________ Date: __________ Organic Nomenclature Quiz Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. ____ 1. Name CH3CH(OH)CH3. a. iso-ethanol b. tertiary-propanol c. butanol d. propanol e. propan-2-ol ____ 2. Which organic compound is unsaturated? a. ethylcyclopentane b. 1-methyl-3-ethylpent-1-yne c. 1,1-dimethylhexane d. cyclohexane e. 1,3,5-trimethyloctane ____ 3. Name the following compound. a. b. c. d. e. ____ 4-ethyl-3-methylheptane 4-methyl-3-propylhexane 3-propyl-4-methylhexane 4-ethyl-3-methylhexene 3-ethyl-4-propylheptane 4. Which alkane would most likely be a liquid at room temperature? a. methane b. ethane c. propane d. butane e. pentane 1 ID: A Name: ________________________ ID: A ____ 5. Which feature do all aromatic hydrocarbons have? a. an amine group b. halogens c. a benzene ring structure d. an aldehyde group e. all double bonds in a ring ____ 6. To which family of organic compounds does CH3COCH2CH2CH3 belong? a. alcohol b. aldehyde c. alkyne d. ketone e. carboxylic acid ____ 7. Name the following compound. a. b. c. d. e. ____ 8. Which compound is a structural isomer of the compound shown below? a. b. c. d. e. ____ 1-methyl-3,4-dibromocyclopentene 3-methyl-1,2-dibromocyclopentene 3-methyl-4,5-dibromocyclopentene 3-methyl-3,4-dibromocyclopentene 2-methyl-3,4-dibromocyclopentene propane butane methane pentane hexane 9. Which class of organic compounds does not contain a carbon-oxygen double bond? a. amides b. ethers c. ketones d. esters e. carboxylic acids 2 Name: ________________________ ID: A ____ 10. What is the difference between an amine and an amide? a. There is no carbon-oxygen bond in an amine, but there is in an amide. b. Amines are non-polar molecules. c. Amines always have a larger molecular weight than amides. d. Amines always have a nitrogen atom attached to two carbon atoms. e. Amines can be found in proteins, but amides can not. ____ 11. Which molecule is polar? a. carbon dioxide b. carbon tetrachloride c. propane d. methyl chloride e. butane ____ 12. What is the shape of an alkyne molecule? a. bent b. linear c. tetrahedral d. trigonal planar e. cyclic ____ 13. To predict whether or not an organic molecule contains a polar bond, what do you not need to do? a. Consider the three-dimensional shape of the molecule b. Consider the bond dipoles within the molecule. c. Determine if there is more than one single bond within the molecule. d. Determine if there is an imbalance in charge within the molecule. e. Use the electronegativity values of each atom to determine the polarity of each bond. ____ 14. When numbering the main chain or ring of an organic compound, which functional group gets the highest priority? a. methyl b. hydroxyl c. halogen d. amine e. hydrogen ____ 15. Which substance is not a structural isomer of hexyne? a. 2-hexyne b. 3-hexyne c. 2,2-dimethylpentyne d. 4-methyl-1-pentyne e. 2,3-dimethylbutadiene ____ 16. Why does the boiling point of an alkane increase as its chain length increases? a. There is more hydrogen bonding. b. There are more hydrogen atoms available for hydrogen bonds to form. c. The number of dipole-dipole interactions increases. d. The strength of the dispersion forces increases with increased molecular size. e. Heavier molecules cannot float on the surface of water as well. 3 Name: ________________________ ID: A ____ 17. Which shape is used to represent cyclobutane? a. triangle b. square c. hexagon d. octagon e. cube ____ 18. How many isomers have the molecular formula C5H10O? a. four b. five c. six d. seven e. three ____ 19. Which two compounds react to form an ester? a. a carboxylic acid and an alcohol b. an alcohol and an aldehyde c. an alcohol and a ketone d. a carboxylic acid and a ketone e. an aldehyde and a carboxylic acid ____ 20. To which class does CH3CH2NH2 belong? a. secondary amine b. primary amine c. secondary amide d. primary amide e. tertiary amine Short Answer 21. Write the structural formula of each compound. a) an alcohol derived from butane b) an aldehyde derived from butane c) an acid derived from butane 4 Name: ________________________ ID: A 22. Explain why propanol is more soluble in water than propane. 23. Explain why an aldehyde with a low molecular weight has a lower melting point and boiling point than an alcohol with a low molecular weight and the same number of carbon atoms. 24. Describe dispersion forces, and explain how they affect the physical properties of organic molecules. Problem 25. Complete the following table. Name 2-fluoro-3,3-dimethylbutanal Complete structural diagram 4-methylpentanamide 1,3-dimethylbenzene 5 Class