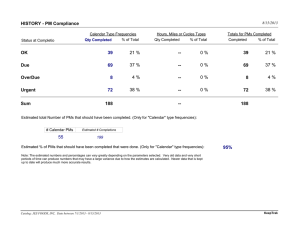
Signature Approvals Name Title Signature Date PMS Report Author(s) Jeffrey Chou Regulatory Affairs Specialist Jeffrey Chou 12/17/2021 PMS Report Reviewers Farhad Daghighian Chief Scientist 12/17/2021 Abbreviations/Acronyms/Definitions Abbreviation/Acronym CAPA CER EU FDA FSCA GHTF GSPRs HHE IMDRF IFU IPU IQS MAUDE MDR MedSun PCR PMCF PMS OUS Q1 TGA Australia TPLC US UK Definition Corrective Action/Preventive Action Clinical Evaluation Report European Union US Food and Drug Administration Field Safety Corrective Action Global Harmonization Task Force General Safety and Performance Requirements Health Hazard Evaluation International Medical Device Regulators Forum Instructions for Use – User Guide Incident Per Unit Initial Quality Study FDA – Manufacturer and User Facility Device Experience Medical Device Regulation FDA – Medical Product Safety Network Reports Problem Cause Resolution Code Post Market Clinical Follow-up Post Market Surveillance Out of United States First quarter of year Therapeutic Goods Administration FDA – Total Product Life Cycle United States United Kingdom 1. Purpose/Objectives of the Post-Market Surveillance (PMS) Report This PMS Report summarizes the systematic collection of post-market regulatory activities and company processes, including metrics, used to continually monitor the safety and performance of the Node Seeker 2000 and to facilitate a comprehensive analysis of the clinical outcomes. The objectives of this PMS Report are as follows: 1. Provide a summary of the collection and review of marketplace experience for this device. 2. Ensure the utmost public safety and well-being exists for patients in a clinical environment. 3. Provide for the health and safety of healthcare professional end users of this device. 4. Summarize collected data and note actions on any necessary preventive or corrective actions. As this is the initial PMS Report developed by Intra-Medical Imaging to comply with the new EU MDR requirements, the data and analysis is from product launch in December 2017 to November 12, 2021. In the future, scheduled reports will be generated on an annual basis within Q1 of each calendar year. 2. Scope The following regulatory, directive, and guidance documents have been reviewed and utilized in preparing the associated Post Market Surveillance Plan 2020 EU MDR 2017/745, Medical Device Regulation (European Parliament and the Council of 5 April 2017) MEDDEV 2.7-1 rev. 4, Medical Device Directive (Council Directive 93/42/EEC of 14 June 1993) MEDDEV 2.12-1 rev. 8, Additional Guidance Regarding the Vigilance Systems as outlined in MEDDEV 2.12-1 rev.8 MEDDEV 2.12-2 rev.2, Guidelines on Medical Devices Post Market Clinical Follow-up Studies EN ISO 13485: 2016, clauses 8.2.1 (Feedback) and 8.2.5 (Monitoring and measurement of processes) NBMED 2.12 rec 1, Coordination of Notified Bodies Medical Devices on Council Directives 90/385/ECC,93/42/EEC and 98/79/EC Post-Marketing Surveillance (PMS) post market/production 3. 3.1 Description of the Product or Product System Product or Product System: Node Seeker 2000: 4. Product(s) Instructions for Use (IFUs) The intended use for Node Seeker 2000 5. Clinical Literature Data Analysis Content included in the Clinical Literature relevant to safety and performance will be discussed in the Clinical Evaluation Report. 6. Internal PMS Data Review and Evaluation The information within this report is being conveyed from manufacturing and engineering systems, quality management systems, marketing, and sales, as well as patients, healthcare professional users, distributors, and importers. 6.1 6.2 6.3 7. CAPA: Corrective and Preventive Action Feedback Complaint data inclusive to relevant Service Request Information