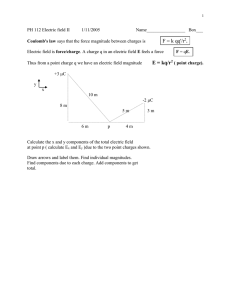
PHY104 ONLINE DERS NOTLARI CHAPTER 23 ELECTRIC FIELD INTRODUCTORY REMARKS Basic knowledge about electricity and magnetism is important for the use and operation of many devices. The electrical force holds the atoms and molecules in all objects included our body. Historical Notes - Chimes observed magnetism as early as 2000 BC. - Anatolians observed the electrical and magnetic phenomena as early as 700 BC on amber and magnetite - William Gilbert showed electrification effects were a general phenomenon not confined to just amber. - Charles Coulomb formulated the electrical force of interaction between the electric charges, 1785 and that law is known as the Coulomb law - Hans Oersted observed that electric current deflects a compass needle placed nearby, (1819). This observation linked- combined the science of electricity and magnetism. - Faraday and Henry showed that when a magnet moves towards a coil of wire, or a coil of wire moves towards a magnet an electric current is established in the coil, (1831). Tis phenomenon is known as the law of induction. - There are four basic laws of electromagnetism. Maxwell used experimental facts and reformulated the laws of electromagnetism, 1873. Electric Charges and Matter - Matter are made of atoms. - Atoms consist of dense small concentrated central part called nucleus and atomic electrons revolving around the nucleus at various energy shells and subshells. - At the nucleus there are proton and neutrons. - Only two kinds of electric charges exist in nature labeled as positive and negative. - Electrons possess negative charges and protons possess positive charges. Neutron do not have charge. The Fundamental Charge - The amount of the charges possessed by the proton and by the electrons are same, that is called the fundamental charge. - The fundamental charge (possessed by the protons and the electrons) is the smallest amount of charge (quantum of charge) exist in isolated form. - These statements show that the concept of charge and matter are closely related. - The electric charges exist in the atom that means, electric charges exist in the all objects and materials. - The positive and negative charges tend to cancel the effects of each other. - An electrically neutral atom has equal number of electrons and protons that specify the atomic number of that atom. - Therefore, normally an object has equal amount of positive and negative charges. such and object appears as charge less when observed from outside. Charging a Matter - Charging a matter means, upsetting charge equality in that matter. - Charge equality may be altered by transfer of electrons from one object to another. Why charge movements do not occur with transfer of neutrons? - If an object loss some electrons by some means, then that object is positively charged. - If an object gains some excess electrons by some means, then that object is negatively charged. - One way to charge materials is by rubbing. For example, a rubber rod rubbed with fur gains electrons and we say it is negatively charged. For example, a glass rod rubbed with silk losses electrons and we say it is positively charged. Read from the textbook and learn how charging may be done by induction. Interracion of Charges - Charges of the same sign repel one another and charges with opposite signs attract one another. - The fundamental charge explained above is, |e| = 1.6x10-19 C, Coulmb. Coulomb’S Law F = k (q1.q2) / r2, Where, F is in Newton (N), q1 and q2 are in Coulomb (C) and r is in meter (m). k = 8.9876 x 109 N.m2 / C2 ~ 9.0 x 109 N.m2 / C2 k = 1 / 4Πε0 ε0 = 8.85x10-12 C2/ N.m2. Read from the textbook the experiments Charles Coulomb and his associates conducted that led the invention of the Coulomb law as stated above. EXAMPLE 1: Find the electrical and gravitational forces between the proton and electron in the hydrogen atom. Distance between proton and electron in H atom is about, 5.3 x 10-11m. FE = k (q1.q2) / r2 = 9.0 x 109 N.m2 / C2 (1.6 x10-19 C)2 / 5.3 x 1011 m)2. FE = 8.2 x 10-8 N. FG = G (m1.m2) / r2 = 6. 67 x 10-11 N.m2 / kg2 (9.11 x10-31 kg)2 / 1.67 x 1027 m)2. FG = 3.6 x 10-47 N. FE / FG ~ 2 x 1039. This result indicates that the electrical force in the atoms much larger than the gravitational force. It is the electrical force that holds the atoms and molecules together in ordinary matter. EXAMPLE 2: On which pair of particles would a force of attraction occur? (a) proton- neutron, (b) electron-neutron, (c) neutron-neutron, (d) proton-proton, (e) proton- electron. EXAMPLE 3: Two point charges, + 5Q and - Q are separated by a distance 2d. (Write your results in terms of k, Q and d. Find the magnitude of the electric interaction force (F) they act to each other. Solution: F = k5Q2/4d2. EXAMPLE 4: Two particles with equal and opposite point charges, (+ q) and (q) are located on the x axis at x = – a, and at x = + a as shown in the figure. (Write your answers in terms of k- Coulomb constant, q and a. Note that the distance between the charges is 2a). Write the magnitude and direction of the electric force (F) acting on the particle at x = -a. Solution: F = kq2/(2a)2 = kq2/4a2 (i), (i) indicate the direction of the force acting on the particle at x = -a. EXAMPLE 5: Refer to the Example 4 above. Suppose a proton of charge +e is located at the origin O(0,0). What is the direction of the total-net electrostatic force on the proton at the origin? Answer: The force on the proton is directed along the + x-axis. Can you write the magnitude of the force in terms of the given parameters? Example 6: Two particles with equal and opposite point charges, (+ q) and (q) are located on the x axis at x = – a, and at x = + a as shown in the figure. . Note that the distance between the charges is 2a. Such two equal and opposite charges separated by a small distance form an electric dipole. Find the electric field on the y-axis at a distance y from the origin in terms of the given parameters. Solution: Refer to the figure and try to solve this example. The Electric Field - The field concept is useful in physics. - The electric field mediates the interactions of the electric charges. Definition of the Electric field First of all, the electric fields are created by the electric charges. Suppose there are electric charges around and we are interested to define the electric field at point P. Assume to place a small positive test charge, q0 at point P, if there is an electrical force acting on the test charge we say that there is an electric field at point P given by, E = F/q0, E is directed along F. The SI unit of E is N/C. Another unit of E, V/m to be defined in Chapter 25. The Electric Field Due to a Point Charge Assume a point charge is located at the origin of a coordinate system. We are interested to find the electric field at point P at a distance r from the point charge. We assume to place a positive test charge q0 at point P. The electrical force acting to the test chare is, F = k (q.q0) / r2, E = F/q0 = k q / r2 F = (1/4π€0).(q / r2) EXAMPLE 7: Two particles with equal and opposite point charges, (+ q) and (q) are located on the x axis at x = – a, and at x = + a as shown in the figure. (Write your answers in terms of k- Coulomb constant, q and a. Note that the distance between the charges is 2a). Write the magnitude and direction of the electric field (E) at the midpoint, at the origin. Solution: E = E1 + E2, E = k q / a2 (i) + k q / a2 (i), The first term is the electric field due to – q charge at the origin and the second term is the electric field due to + q charge at the origin. E = 2 k q / a2 (i). EXAMPLE 8: Four identical point charges are located at the corner of a square. What is the electric field at the midpoint, the intersection of the diagonals of the square. Answer: E = 0, why? Electric Field Lines To be explained during the lecture. Electric Fields due to Continuous Charge Distributions To be explained during the lecture. Motion of Charged Particles in an Electric Field Recal the definition of the electric field, E = F/q0, From the above equation; F = qe = ma a = qe/m Example 9: A particle having charge q = 6.4x10-6 C is in a uniform electric field. The electrical force acting on the particle is F = 36 i N, where i is the unit vector along the +x axis (a) Find the electric field? (b) If the mass of the particle is 9 x 10-3 kg what is its acceleration? Answers: (a) E = 5.6x106 N/C (i) (b) a = 4x103 m/s2 (i) Example 10: A particle has charge, q = 6.4x10-6 C. How many electrons are missing in the particle? (e = 1.6 x 10-19 C). Answer: Q = n |e| n = 6.4x10-6 C/ 1.6 x 10-19 C = 4 x 1013 electrons.