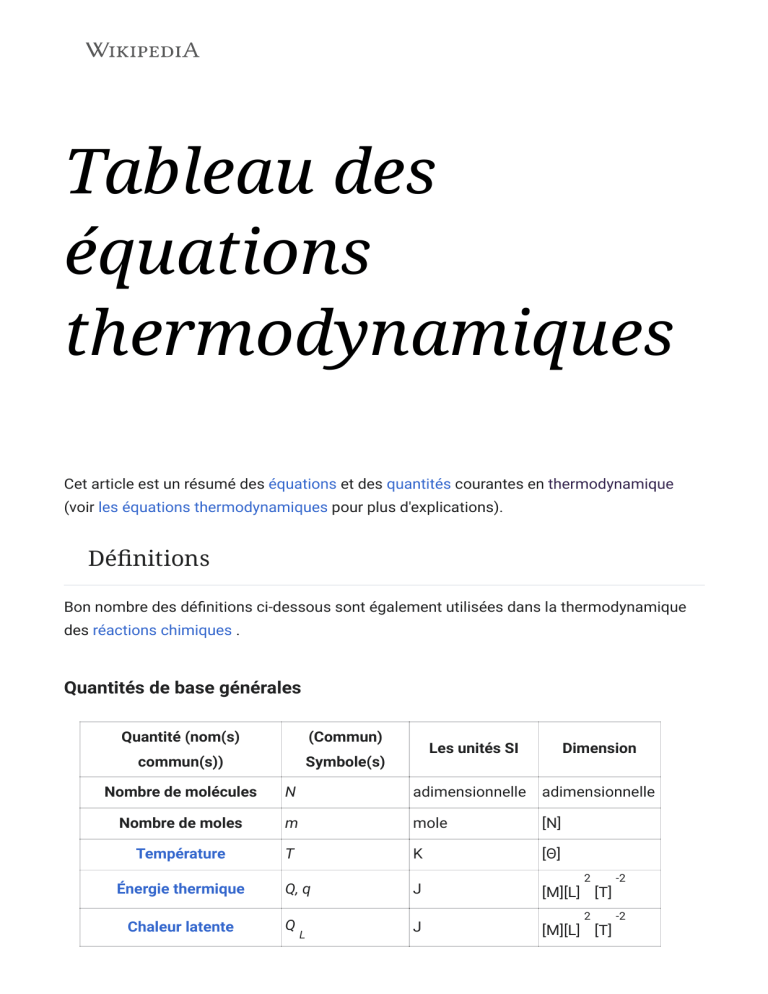
Tableau des équations thermodynamiques Cet article est un résumé des équations et des quantités courantes en thermodynamique (voir les équations thermodynamiques pour plus d'explications). Définitions Bon nombre des définitions ci-dessous sont également utilisées dans la thermodynamique des réactions chimiques . Quantités de base générales Quantité (nom(s) (Commun) commun(s)) Symbole(s) Les unités SI Dimension Nombre de molécules N adimensionnelle adimensionnelle Nombre de moles m mole [N] Température T K [Θ] Q, q J [M][L] [T] Q J [M][L] [T] Énergie thermique Chaleur latente L 2 -2 2 -2 Grandeurs dérivées générales Quantité (nom(s) (Commun) Définition de commun(s)) Symbole(s) l'équation Les unités SI Bêta Dimension -1 thermodynamique , ?? 2 -1 -2 J [T] [M] [L] J 2 -2 [M] [L] [T] Température inverse Température thermodynamique ?? 2 Entropie -1 S JK , -2 [M][L] [T] -1 -2 Pression P Pennsylvanie ML Énergie interne U J [M][L] [T] Enthalpie H J Fonction de partition Z L'énergie gratuite de Gibbs J J i (de composant i dans un mélange) T 2 2 -2 -2 [M][L] [T] adimensionnelle adimensionnelle g Potentiel chimique μ [Θ] -1 , où F n'est pas proportionnel à N car μ dépend de i la pression. , où G est proportionnel à N (tant que la composition du 2 -2 [M][L] [T] 2 [M][L] [T] -2 rapport molaire du système reste la même) car μ i ne dépend que de la température, de la pression et de la composition. L'énergie libre de Helmholtz UN F 2 J [M][L] [T] J [M][L] [T] Potentiel Landau , Landau Free Energy, Ω , Φ G 2 -2 -2 Grand potentiel 2 Potentiel de Massieu, entropie -1 ?? JK ?? J K−1 [M][L] [T] -2 [Θ] -1 libre de Helmholtz Potentiel de Planck, entropie libre de Gibbs Thermal properties of matter [M][L]2[T]−2 [Θ]−1 Quantity (common (Common) name/s) symbol/s General heat/thermal capacity Heat capacity (isobaric) Specific heat capacity (isobaric) Molar specific heat capacity (isobaric) Heat capacity (isochoric/volumetric) Specific heat capacity (isochoric) Molar specific heat capacity (isochoric) Specific latent heat Defining equation SI units Dimension [M][L]2[T]−2 [Θ] C J K −1 Cp J K −1 Cmp J kg−1 K−1 Cnp J K −1 mol−1 CV J K −1 CmV J kg−1 K−1 CnV JK −1 L J kg−1 γ dimensionless dimensionless −1 [M][L]2[T]−2 [Θ] −1 [L]2[T]−2 [Θ]−1 [M][L]2[T]−2 [Θ] −1 [N]−1 [M][L]2[T]−2 [Θ] −1 mol−1 [L]2[T]−2 [Θ]−1 [M][L]2[T]−2 [Θ] −1 [N]−1 [L]2[T]−2 Ratio of isobaric to isochoric heat capacity, heat capacity ratio, adiabatic index Thermal transfer Quantity (common (Common) name/s) symbol/s Temperature No standard gradient Defining equation symbol SI units Dimension -1 K m−1 [Θ][L] Taux de conduction thermique, courant thermique, thermique/ flux P -1 W=Js 2 [M] [L] [T] thermique, transfert de puissance thermique Intensité thermique −2 je Wm q Wm -3 [M] [T] Densité de flux thermique/chaleur (analogue vectoriel de l'intensité thermique cidessus) Équations Les équations de cet article sont classées par sujet. Processus thermodynamiques −2 -3 [M] [T] -3 Situation physique Équations Pour un gaz parfait Processus isentropique (adiabatique et réversible) Processus isotherme Pour un gaz parfait p = p , p = constant 1 2 Processus isobare V = V , V = constante 1 2 Processus isochore Extension gratuite Traiter Travail effectué par un gaz en expansion Travail net effectué dans des processus cycliques Théorie cinétique Équations des gaz parfaits Situation physique Nomenclature p = pression V = volume du conteneur T = température Loi des gaz parfaits n = nombre de moles R = Constante de gaz N = nombre de molécules k = constante de Boltzmann Pression d'un gaz parfait Gaz parfait m = masse d' une molécule M m = masse molaire Équations Quantité Équation Isobare Isochore Isotherme générale Δp=0 ΔV=0 ΔT=0 (pour gaz (pour gaz parfait parfait Adiabatique Travail W Capacité calorifique C (comme pour monoatomique) monoatomique) le vrai gaz) (pour le gaz (pour le gaz parfait parfait diatomique) diatomique) L' énergie interne ΔU Enthalpie ΔH Entropie Δs [1] Constant Entropy , where kB is the Boltzmann constant, and Ω denotes the volume of macrostate in the phase space or otherwise called thermodynamic probability. , for reversible processes only Statistical physics Below are useful results from the Maxwell–Boltzmann distribution for an ideal gas, and the implications of the Entropy quantity. The distribution is valid for atoms or molecules constituting ideal gases. Physical situation Nomenclature Equations v = velocity of atom/molecule, Non-relativistic m = mass of each molecule (all molecules are speeds identical in kinetic theory), γ(p) = Lorentz factor as function of Maxwell–Boltzmann momentum (see below) distribution Ratio of thermal to rest mass-energy of each molecule: Relativistic speeds (Maxwell-Jüttner distribution) K2 is the Modified Bessel function of the second kind. Entropy Logarithm P = probability of system in microstate i i of the density of Ω = total number of microstates states where: Entropy change Entropic force Average kinetic energy per degree of freedom Equipartition theorem df = degree of freedom Internal energy Corollaries of the non-relativistic Maxwell–Boltzmann distribution are below. Physical situation Nomenclature Equations Mean speed Root mean square speed Modal speed σ = Effective cross-section Mean free path n = Volume density of number of target particles ℓ = Mean free path Quasi-static and reversible processes For quasi-static and reversible processes, the first law of thermodynamics is: where δQ is the heat supplied to the system and δW is the work done by the system. Thermodynamic potentials The following energies are called the thermodynamic potentials, Name Symbol Formula Natural variables Internal energy Helmholtz free energy Enthalpy Gibbs free energy Landau potential, or grand potential , and the corresponding fundamental thermodynamic relations or "master equations"[2] are: Potential Differential Internal energy Enthalpy Helmholtz free energy Gibbs free energy Maxwell's relations The four most common Maxwell's relations are: Physical situation Nomenclature = Internal energy Thermodynamic potentials as functions of their natural variables = Enthalpy = Helmholtz free energy = Gibbs free energy More relations include the following. Other differential equations are: Equations Name H U G Gibbs–Helmholtz equation Quantum properties Indistinguishable Particles où N est le nombre de particules, h est la constante de Planck , I est le moment d'inertie et Z est la fonction de partition , sous diverses formes : Degré de liberté Fonction de partition Traduction Vibration Rotation où: σ = 1 ( molécules hétéronucléaires ) σ = 2 ( homonucléaire ) Propriétés thermiques de la matière Coefficients Joule-Thomson coefficient Compressibility (constant temperature) Coefficient of thermal expansion (constant pressure) Heat capacity (constant pressure) Heat capacity (constant volume) Derivation of heat capacity (constant pressure) Since Equation Derivation of heat capacity (constant volume) Since (where δWrev is the work done by the system), Thermal transfer Physical situation Nomenclature Equations Texternal = external temperature (outside of system) Net intensity emission/absorption Tsystem = internal temperature (inside system) ε = emmisivity Internal energy of a substance CV = isovolumetric heat capacity of substance ΔT = temperature change of substance Cp = isobaric heat capacity Meyer's equation CV = isovolumetric heat capacity n = number of moles Series Effective thermal conductivities λi = thermal conductivity of substance i λnet = equivalent thermal conductivity Parallel Thermal efficiencies Physical situation Nomenclature Equations η = efficiency W = work done by engine Thermodynamic engine: QH = heat energy in higher temperature Thermodynamic engines reservoir QL = heat energy in lower temperature reservoir Carnot engine efficiency: TH = temperature of higher temp. reservoir TL = temperature of lower temp. reservoir Refrigeration performance Refrigeration K = coefficient of refrigeration performance Carnot refrigeration performance Voir également Antoine equation Bejan number Bowen ratio Bridgman's equations Clausius–Clapeyron relation Departure functions Duhem–Margules equation Ehrenfest equations Gibbs–Helmholtz equation Gibbs' phase rule Kopp's law Kopp–Neumann law Noro–Frenkel law of corresponding states Onsager reciprocal relations Stefan number Triple product rule Exact differential Les références 1. Keenan, Thermodynamics, Wiley, New York, 1947 2. Physical chemistry, P.W. Atkins, Oxford University Press, 1978, ISBN 0 19 855148 7 Atkins, Peter and de Paula, Julio Physical Chemistry, 7th edition, W.H. Freeman and Company, 2002 ISBN 0-7167-3539-3. Chapters 1–10, Part 1: "Equilibrium". Bridgman, P. W. (1 March 1914). "A Complete Collection of Thermodynamic Formulas" (https://babel. hathitrust.org/cgi/pt?id=uc1.31210014450082&view=1up&seq=289) . Physical Review. American Physical Society (APS). 3 (4): 273–281. doi:10.1103/physrev.3.273 (https://doi.org/10.1103%2Fphysr ev.3.273) . ISSN 0031-899X (https://www.worldcat.org/issn/0031-899X) . Landsberg, Peter T. Thermodynamics and Statistical Mechanics. New York: Dover Publications, Inc., 1990. (reprinted from Oxford University Press, 1978). Lewis, G.N., and Randall, M., "Thermodynamics", 2nd Edition, McGraw-Hill Book Company, New York, 1961. Reichl, L.E., A Modern Course in Statistical Physics, 2nd edition, New York: John Wiley & Sons, 1998. Schroeder, Daniel V. Thermal Physics. San Francisco: Addison Wesley Longman, 2000 ISBN 0-20138027-7. Silbey, Robert J., et al. Physical Chemistry, 4th ed. New Jersey: Wiley, 2004. Callen, Herbert B. (1985). Thermodynamics and an Introduction to Themostatistics, 2nd edition, New York: John Wiley & Sons. Liens externes



