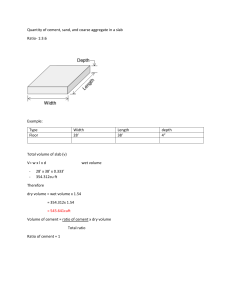
LEARNING GUIDE Week No.: 6_ TOPIC/S CEMENT -Definition -History -Classification of Cement -Portland cement -Composition of Portland cement -Types of Portland cement -Manufacture of Portland cement EXPECTED COMPETENCIES At the end of the lesson, students must be able to: 1. define cement; 2. outline the history of cement; 3. identify the classification, composition and uses/applications of cement and 4. discuss how Portland cement is manufactured. CONTENT Introduction Cement, in general, adhesive substances of all kinds, but, in a narrower sense, the binding materials used in building and civil engineering construction. Cements of this kind are finely ground powders that, when mixed with water, set to a hard mass. Setting and hardening result from hydration, which is a chemical combination of the cement compounds with water that yields submicroscopic crystals or a gel-like material with a high surface area. Because of their hydrating properties, constructional cements, which will even set and harden under water, are often called hydraulic cements. The most important of these is Portland cement. History of Cement The origin of hydraulic cements goes back to ancient Greece and Rome. The materials used were lime and a volcanic ash that slowly reacted with it in the presence of water to form a hard mass. This formed the cementing material of the Roman mortars and concretes of 2,000 years ago and of subsequent construction work in western Europe. Volcanic ash mined near what is now the city of Pozzuoli, Italy, was particularly rich in essential aluminosilicate minerals, giving rise to the classic pozzolana cement of the Roman era. To this day the term pozzolana, or pozzolan, refers either to the cement itself or to any finely divided aluminosilicate that reacts with lime in water to form cement. (The term cement, meanwhile, derives from the Latin word caementum, which meant stone chippings such as were used in Roman mortar—not the binding material itself.) Portland cement is a successor to a hydraulic lime that was first developed by John Smeaton in 1756 when he was called in to erect the Eddystone Lighthouse off the coast of Plymouth, Devon, England. The next development, taking place about 1800 in England and France, was a material obtained by burning nodules of clayey limestone. Soon afterward in the United States, a similar material was obtained by burning a naturally occurring substance called “cement rock.” These materials belong to a class known as natural cement, allied to portland cement but more lightly burned and not of controlled composition. The invention of portland cement usually is attributed to Joseph Aspdin of Leeds, Yorkshire, England, who in 1824 took out a patent for a material that was produced from a synthetic mixture of limestone and clay. He called the product “portland cement” because of a fancied resemblance of the material, when set, to portland stone, a limestone used for building in England. Aspdin’s product may well have been too lightly burned to be a true portland cement, and the real prototype was perhaps that produced by Isaac Charles Johnson in southeastern England about 1850. The manufacture of portland cement rapidly spread to other European countries and North America. During the 20th century, cement manufacture spread worldwide. By the early 21st century, China and India had become the world leaders in cement production, followed by the United States, Brazil, Turkey, and Iran. Cement materials can be classified into two distinct categories: non-hydraulic cements and hydraulic cements according to their respective setting and hardening mechanisms. Hydraulic cements setting and hardening involve hydration reactions and therefore require water, while non-hydraulic cements only react with a gas and can directly set under air. Classification of Cement 1.) Non-hydraulic cement, such as slaked lime (calcium oxide mixed with water), hardens by carbonation in contact with carbon dioxide, which is present in the air (~ 412 vol. ppm ≃ 0.04 vol. %). First calcium oxide (lime) is produced from calcium carbonate (limestone or chalk) by calcination at temperatures above 825 °C (1,517 °F) for about 10 hours at atmospheric pressure: CaCO3 → CaO + CO2 The calcium oxide is then spent (slaked) mixing it with water to make slaked lime (calcium hydroxide): CaO + H2O → Ca(OH)2 Once the excess water is completely evaporated (this process is technically called setting), the carbonation starts: Ca(OH)2 + CO2 → CaCO3 + H2O This reaction takes time, because the partial pressure of carbon dioxide in the air is low (~ 0.4 millibar). The carbonation reaction requires that the dry cement be exposed to air, so the slaked lime is a non-hydraulic cement and cannot be used under water. This process is called the lime cycle. 2.) Hydraulic cement hardens by hydration of the clinker minerals when water is added. Hydraulic cements (such as Portland cement) are made of a mixture of silicates and oxides, the four main mineral phases of the clinker, abbreviated in the cement chemist notation, being: C3S: Alite (3CaO·SiO2); C2S: Belite (2CaO·SiO2); C3A: Tricalcium aluminate (3CaO·Al2O3) (historically, and still occasionally, called 'celite'); C4AF: Brownmillerite (4CaO·Al2O3·Fe2O3). The silicates are responsible for the cement's mechanical properties — the tricalcium aluminate and brownmillerite are essential for the formation of the liquid phase during the sintering (firing) process of clinker at high temperature in the kiln. The chemistry of these reactions is not completely clear and is still the object of research. Applications of Cement Cements may be used alone (i.e., “neat,” as grouting materials), but the normal use is in mortar and concrete in which the cement is mixed with inert material known as aggregate. Mortar is cement mixed with sand or crushed stone that must be less than approximately 5 mm (3/16 inch) in size. Concrete is a mixture of cement, sand or other fine aggregate, and a coarse aggregate that for most purposes is up to 19 to 25 mm (3/4 to 1 inch) in size, but the coarse aggregate may also be as large as 150 mm (6 inches) when concrete is placed in large masses such as dams. Mortars are used for binding bricks, blocks, and stone in walls or as surface renderings. Concrete is used for a large variety of constructional purposes. Mixtures of soil and portland cement are used as a base for roads. Portland cement also is used in the manufacture of bricks, tiles, shingles, pipes, beams, railroad ties, and various extruded products. The products are prefabricated in factories and supplied ready for installation. Because concrete is the most widely used of all construction materials in the world today, the manufacture of cement is widespread. Each year almost one ton of concrete is poured per capita in the developed countries. Raw Materials Composition Portland cement consists essentially of compounds of lime (calcium oxide, CaO) mixed with silica (silicon dioxide, SiO2) and alumina (aluminum oxide, Al2O3). The lime is obtained from a calcareous (lime-containing) raw material, and the other oxides are derived from an argillaceous (clayey) material. Additional raw materials such as silica sand, iron oxide (Fe2O3), and bauxite—containing hydrated aluminum, Al(OH)3—may be used in smaller quantities to get the desired composition. The commonest calcareous raw materials are limestone and chalk, but others, such as coral or shell deposits, also are used. Clays, shales, slates, and estuarine muds are the common argillaceous raw materials. Marl, a compact calcareous clay, and cement rock contain both the calcareous and argillaceous components in proportions that sometimes approximate cement compositions. Another raw material is blast-furnace slag, which consists mainly of lime, silica, and alumina and is mixed with a calcareous material of high lime content. Kaolin, a white clay that contains little iron oxide, is used as the argillaceous component for white portland cement. Industrial wastes, such as fly ash and calcium carbonate from chemical manufacture, are other possible raw materials, but their use is small compared with that of the natural materials. The magnesia (magnesium oxide, MgO) content of raw materials must be low because the permissible limit in portland cement is 4 to 5 percent. Other impurities in raw materials that must be strictly limited are fluorine compounds, phosphates, metal oxides and sulfides, and excessive alkalies. Another essential raw material is gypsum, some 5 percent of which is added to the burned cement clinker during grinding to control the setting time of the cement. Portland cement also can be made in a combined process with sulfuric acid using calcium sulfate or anhydrite in place of calcium carbonate. The sulfur dioxide produced in the flue gases on burning is converted to sulfuric acid by normal processes. Extraction and processing Raw materials employed in the manufacture of cement are extracted by quarrying in the case of hard rocks such as limestones, slates, and some shales, with the aid of blasting when necessary. Some deposits are mined by underground methods. Softer rocks such as chalk and clay can be dug directly by excavators. The excavated materials are transported to the crushing plant by trucks, railway freight cars, conveyor belts, or ropeways. They also can be transported in a wet state or slurry by pipeline. In regions where limestones of sufficiently high lime content are not available, some process of beneficiation can be used. Froth flotation will remove excess silica or alumina and so upgrade the limestone, but it is a costly process and is used only when unavoidable. Manufacture of Cement The cement-making process, from crushing and grinding of raw materials, through roasting of the ground and mixed ingredients, to final cooling and storing of the finished product. There are four stages in the manufacture of portland cement: (1) crushing and grinding the raw materials, (2) blending the materials in the correct proportions, (3) burning the prepared mix in a kiln, and (4) grinding the burned product, known as “clinker,” together with some 5 percent of gypsum (to control the time of set of the cement). The three processes of manufacture are known as the wet, dry, and semidry processes and are so termed when the raw materials are ground wet and fed to the kiln as a slurry, ground dry and fed as a dry powder, or ground dry and then moistened to form nodules that are fed to the kiln. Crushing and grinding All except soft materials are first crushed, often in two stages, and then ground, usually in a rotating, cylindrical ball, or tube mills containing a charge of steel grinding balls. This grinding is done wet or dry, depending on the process in use, but for dry grinding the raw materials first may need to be dried in cylindrical, rotary dryers. Soft materials are broken down by vigorous stirring with water in wash mills, producing a fine slurry, which is passed through screens to remove oversize particles. Blending A first approximation of the chemical composition required for a particular cement is obtained by selective quarrying and control of the raw material fed to the crushing and grinding plant. Finer control is obtained by drawing material from two or more batches containing raw mixes of slightly different composition. In the dry process these mixes are stored in silos; slurry tanks are used in the wet process. Thorough mixing of the dry materials in the silos is ensured by agitation and vigorous circulation induced by compressed air. In the wet process the slurry tanks are stirred by mechanical means or compressed air or both. The slurry, which contains 35 to 45 percent water, is sometimes filtered, reducing the water content to 20 to 30 percent, and the filter cake is then fed to the kiln. This reduces the fuel consumption for burning. Burning The earliest kilns in which cement was burned in batches were bottle kilns, followed by chamber kilns and then by continuous shaft kilns. The shaft kiln in a modernized form is still used in some countries, but the dominant means of burning is the rotary kiln. These kilns— up to 200 metres (660 feet) long and six metres in diameter in wet process plants but shorter for the dry process—consist of a steel, cylindrical shell lined with refractory materials. They rotate slowly on an axis that is inclined a few degrees to the horizontal. The raw material feed, introduced at the upper end, moves slowly down the kiln to the lower, or firing, end. The fuel for firing may be pulverized coal, oil, or natural gas injected through a pipe. The temperature at the firing end ranges from about 1,350 to 1,550 °C (2,460 to 2,820 °F), depending on the raw materials being burned. Some form of heat exchanger is commonly incorporated at the back end of the kiln to increase heat transfer to the incoming raw materials and so reduce the heat lost in the waste gases. The burned product emerges from the kiln as small nodules of clinker. These pass into coolers, where the heat is transferred to incoming air and the product cooled. The clinker may be immediately ground to cement or stored in stockpiles for later use. In the semidry process the raw materials, in the form of nodules containing 10 to 15 percent water, are fed onto a traveling chain grate before passing to the shorter rotary kiln. Hot gases coming from the kiln are sucked through the raw nodules on the grate, preheating the nodules. Dust emission from cement kilns can be a serious nuisance. In populated areas it is usual and often compulsory to fit cyclone arrestors, bag-filter systems, or electrostatic dust precipitators between the kiln exit and the chimney stack. Modern cement plants are equipped with elaborate instrumentation for control of the burning process. Raw materials in some plants are sampled automatically, and a computer calculates and controls the raw mix composition. The largest rotary kilns have outputs exceeding 5,000 tons per day. Grinding The clinker and the required amount of gypsum are ground to a fine powder in horizontal mills similar to those used for grinding the raw materials. The material may pass straight through the mill (open-circuit grinding), or coarser material may be separated from the ground product and returned to the mill for further grinding (closed-circuit grinding). Sometimes a small amount of a grinding aid is added to the feed material. For air-entraining cements (discussed in the following section) the addition of an air-entraining agent is similarly made. Finished cement is pumped pneumatically to storage silos from which it is drawn for packing in paper bags or for dispatch in bulk containers. THE MAJOR CEMENTS: COMPOSITION AND PROPERTIES Portland cement Chemical composition Portland cement is made up of four main compounds: tricalcium silicate (3CaO · SiO2), dicalcium silicate (2CaO · SiO2), tricalcium aluminate (3CaO · Al2O3), and a tetracalcium aluminoferrite (4CaO · Al2O3Fe2O3). In an abbreviated notation differing from the normal atomic symbols, these compounds are designated as C3S, C2S, C3A, and C4AF, where C stands for calcium oxide (lime), S for silica, A for alumina, and F for iron oxide. Small amounts of uncombined lime and magnesia also are present, along with alkalies and minor amounts of other elements. Hydration The most important hydraulic constituents are the calcium silicates, C2S and C3S. Upon mixing with water, the calcium silicates react with water molecules to form calcium silicate hydrate (3CaO · 2SiO2 · 3H2O) and calcium hydroxide (Ca[OH]2). These compounds are given the shorthand notations C–S–H (represented by the average formula C3S2H3) and CH, and the hydration reaction can be crudely represented by the following reactions:2C3S + 6H = C3S2H3 + 3CH2C2S + 4H = C3S2H3 + CH During the initial stage of hydration, the parent compounds dissolve, and the dissolution of their chemical bonds generates a significant amount of heat. Then, for reasons that are not fully understood, hydration comes to a stop. This quiescent, or dormant, period is extremely important in the placement of concrete. Without a dormant period there would be no cement trucks; pouring would have to be done immediately upon mixing. Following the dormant period (which can last several hours), the cement begins to harden, as CH and C–S–H are produced. This is the cementitious material that binds cement and concrete together. As hydration proceeds, water and cement are continuously consumed. Fortunately, the C–S–H and CH products occupy almost the same volume as the original cement and water; volume is approximately conserved, and shrinkage is manageable. Although the formulas above treat C–S–H as a specific stoichiometry, with the formula C3S2H3, it does not at all form an ordered structure of uniform composition. C–S–H is actually an amorphous gel with a highly variable stoichiometry. The ratio of C to S, for example, can range from 1:1 to 2:1, depending on mix design and curing conditions. Structural properties The strength developed by portland cement depends on its composition and the fineness to which it is ground. The C3S is mainly responsible for the strength developed in the first week of hardening and the C2S for the subsequent increase in strength. The alumina and iron compounds that are present only in lesser amounts make little direct contribution to strength. Set cement and concrete can suffer deterioration from attack by some natural or artificial chemical agents. The alumina compound is the most vulnerable to chemical attack in soils containing sulfate salts or in seawater, while the iron compound and the two calcium silicates are more resistant. Calcium hydroxide released during the hydration of the calcium silicates is also vulnerable to attack. Because cement liberates heat when it hydrates, concrete placed in large masses, as in dams, can cause the temperature inside the mass to rise as much as 40 °C (70 °F) above the outside temperature. Subsequent cooling can be a cause of cracking. The highest heat of hydration is shown by C3A, followed in descending order by C3S, C4AF, and C2S. Types of Portland Cement Five types of portland cement are standardized in the United States by the American Society for Testing and Materials (ASTM): ordinary (Type I), modified (Type II), high-earlystrength (Type III), low-heat (Type IV), and sulfate-resistant (Type V). In other countries Type II is omitted, and Type III is called rapid-hardening. Type V is known in some European countries as Ferrari cement. There also are various other special types of portland cement. Coloured cements are made by grinding 5 to 10 percent of suitable pigments with white or ordinary gray portland cement. Air-entraining cements are made by the addition on grinding of a small amount, about 0.05 percent, of an organic agent that causes the entrainment of very fine air bubbles in a concrete. This increases the resistance of the concrete to freeze-thaw damage in cold climates. The air-entraining agent can alternatively be added as a separate ingredient to the mix when making the concrete. Low-alkali cements are portland cements with a total content of alkalies not above 0.6 percent. These are used in concrete made with certain types of aggregates that contain a form of silica that reacts with alkalies to cause an expansion that can disrupt a concrete. Masonry cements are used primarily for mortar. They consist of a mixture of portland cement and ground limestone or other filler together with an air-entraining agent or a water-repellent additive. Waterproof cement is the name given to a portland cement to which a water-repellent agent has been added. Hydrophobic cement is obtained by grinding portland cement clinker with a film-forming substance such as oleic acid in order to reduce the rate of deterioration when the cement is stored under unfavourable conditions. Oil-well cements are used for cementing work in the drilling of oil wells where they are subject to high temperatures and pressures. They usually consist of portland or pozzolanic cement (see below) with special organic retarders to prevent the cement from setting too quickly. Slag Cements The granulated slag made by the rapid chilling of suitable molten slags from blast furnaces forms the basis of another group of constructional cements. A mixture of portland cement and granulated slag, containing up to 65 percent slag, is known in the English-speaking countries as portland blast-furnace (slag) cement. The German Eisenportlandzement and Hochofenzement contain up to 40 and 85 percent slag, respectively. Mixtures in other proportions are found in French-speaking countries under such names as ciment portland de fer, ciment métallurgique mixte, ciment de haut fourneau, and ciment de liatier au clinker. Properties of these slag cements are broadly similar to those of portland cement, but they have a lower lime content and a higher silica and alumina content. Those with the higher slag content have an increased resistance to chemical attack. Another type of slag-containing cement is a supersulfated cement consisting of granulated slag mixed with 10 to 15 percent hard-burned gypsum or anhydrite (natural anhydrous calcium sulfate) and a few percent of portland cement. The strength properties of supersulfated cement are similar to those of portland cement, but it has an increased resistance to many forms of chemical attack. Pozzolanic cements are mixtures of portland cement and a pozzolanic material that may be either natural or artificial. The natural pozzolanas are mainly materials of volcanic origin but include some diatomaceous earths. Artificial materials include fly ash, burned clays, and shales. Pozzolanas are materials that, though not cementitious in themselves, contain silica (and alumina) in a reactive form able to combine with lime in the presence of water to form compounds with cementitious properties. Mixtures of lime and pozzolana still find some application but largely have been superseded by the modern pozzolanic cement. Hydration of the portland cement fraction releases the lime required to combine with the pozzolana. High-Alumina Cement High-alumina cement is a rapid-hardening cement made by fusing at 1,500 to 1,600 °C (2,730 to 2,910 °F) a mixture of bauxite and limestone in a reverberatory or electric furnace or in a rotary kiln. It also can be made by sintering at about 1,250 °C (2,280 °F). Suitable bauxites contain 50 to 60 percent alumina, up to 25 percent iron oxide, not more than 5 percent silica, and 10 to 30 percent water of hydration. The limestone must contain only small amounts of silica and magnesia. The cement contains 35 to 40 percent lime, 40 to 50 percent alumina, up to 15 percent iron oxides, and preferably not more than about 6 percent silica. The principal cementing compound is calcium aluminate (CaO · Al2O3). High-alumina cement gains a high proportion of its ultimate strength within 24 hours and has a high resistance to chemical attack. It also can be used in refractory linings for furnaces. A white form of the cement, containing minimal proportions of iron oxide and silica, has outstanding refractory properties. Expanding and Non-shrinking Cements Expanding and nonshrinking cements expand slightly on hydration, thus offsetting the small contraction that occurs when fresh concrete dries for the first time. Expanding cements were first produced in France about 1945. The American type is a mixture of portland cement and an expansive agent made by clinkering a mix of chalk, bauxite, and gypsum. Gypsum Plasters Gypsum plasters are used for plastering, the manufacture of plaster boards and slabs, and in one form of floor-surfacing material. These gypsum cements are mainly produced by heating natural gypsum (calcium sulfate dihydrate, CaSO4 · 2H2O) and dehydrating it to give calcium sulfate hemihydrate (CaSO4 · 1/2H2O) or anhydrous (water-free) calcium sulfate. Gypsum and anhydrite obtained as by-products in chemical manufacture also are used as raw materials. The hemihydrate, known as plaster of Paris, sets within a few minutes on mixing with water; for building purposes a retarding agent, normally keratin, a protein, is added. The anhydrous calcium sulfate plasters are slower-setting, and often another sulfate salt is added in small amounts as an accelerator. Flooring plaster, originally known by its German title of Estrich Gips, is of the anhydrous type. Physical Properties of Cement Different blends of cement used in construction are characterized by their physical properties. Some key parameters control the quality of cement. The physical properties of good cement are based on: • • • • • • • • • Fineness of cement Soundness Consistency Strength Setting time Heat of hydration Loss of ignition Bulk density Specific gravity (Relative density) Fineness of Cement The size of the particles of the cement is its fineness. The required fineness of good cement is achieved through grinding the clinker in the last step of cement production process. As hydration rate of cement is directly related to the cement particle size, fineness of cement is very important. Soundness of Cement. Soundness refers to the ability of cement to not shrink upon hardening. Good quality cement retains its volume after setting without delayed expansion, which is caused by excessive free lime and magnesia. Consistency of Cement. The ability of cement paste to flow is consistency. It is measured by Vicat Test. Strength of Cement. Three types of strength of cement are measured – compressive, tensile and flexural. Various factors affect the strength, such as water-cement ratio, cement-fine aggregate ratio, curing conditions, size and shape of a specimen, the manner of molding and mixing, loading conditions and age. While testing the strength, the following should be considered: • • • Cement mortar strength and cement concrete strength are not directly related. Cement strength is merely a quality control measure. The tests of strength are performed on cement mortar mix, not on cement paste. Cement gains strength over time, so the specific time of performing the test should be mentioned. Compressive Strength. It is the most common strength test. A test specimen (50mm) is taken and subjected to a compressive load until failure. The loading sequence must be within 20 seconds and 80 seconds. Tensile strength. Though this test used to be common during the early years of cement production, now it does not offer any useful information about the properties of cement. Flexural strength. This is actually a measure of tensile strength in bending. The test is performed in a 40 x40 x 160 mm cement mortar beam, which is loaded at its center point until failure. Setting Time of Cement. Cement sets and hardens when water is added. This setting time can vary depending on multiple factors, such as fineness of cement, cement-water ratio, chemical content, and admixtures. Cement used in construction should have an initial setting time that is not too low and a final setting time not too high. Hence, two setting times are measured: • • Initial set: When the paste begins to stiffen noticeably (typically occurs within 30-45 minutes) Final set: When the cement hardens, being able to sustain some load (occurs below 10 hours) Again, setting time can also be an indicator of hydration rate. Heat of Hydration When water is added to cement, the reaction that takes place is called hydration. Hydration generates heat, which can affect the quality of the cement and also be beneficial in maintaining curing temperature during cold weather. On the other hand, when heat generation is high, especially in large structures, it may cause undesired stress. The heat of hydration is affected most by C3S and C3A present in cement, and also by water-cement ratio, fineness and curing temperature. The heat of hydration of Portland cement is calculated by determining the difference between the dry and the partially hydrated cement (obtained by comparing these at 7th and 28th days). Loss of Ignition Heating a cement sample at 900 - 1000°C (that is, until a constant weight is obtained) causes weight loss. This loss of weight upon heating is calculated as loss of ignition. Improper and prolonged storage or adulteration during transport or transfer may lead to pre-hydration and carbonation, both of which might be indicated by increased loss of ignition. When cement is mixed with water, the water replaces areas where there would normally be air. Because of that, the bulk density of cement is not very important. Cement has a varying range of density depending on the cement composition percentage. The density of cement may be anywhere from 62 to 78 pounds per cubic foot. Specific Gravity (Relative Density) Specific gravity is generally used in mixture proportioning calculations. Portland cement has a specific gravity of 3.15, but other types of cement (for example, portland-blast-furnace-slag and portland-pozzolan cement) may have specific gravities of about 2.90. CHEMICAL PROPERTIES OF CEMENT The raw materials for cement production are limestone (calcium), sand or clay (silicon), bauxite (aluminum) and iron ore, and may include shells, chalk, marl, shale, clay, blast furnace slag, slate. Chemical analysis of cement raw materials provides insight into the chemical properties of cement. 1. Tricalcium aluminate (C3A) Low content of C3A makes the cement sulfate-resistant. Gypsum reduces the hydration of C3A, which liberates a lot of heat in the early stages of hydration. C3A does not provide any more than a little amount of strength. Type I cement: contains up to 3.5% SO3 (in cement having more than 8% C3A) Type II cement: contains up to 3% SO3 (in cement having less than 8% C3A) 2. Tricalcium silicate (C3S) C3S causes rapid hydration as well as hardening and is responsible for the cement’s early strength gain an initial setting. 3. Dicalcium silicate (C2S) As opposed to tricalcium silicate, which helps early strength gain, dicalcium silicate in cement helps the strength gain after one week. 4. Ferrite (C4AF) Ferrite is a fluxing agent. It reduces the melting temperature of the raw materials in the kiln from 3,000°F to 2,600°F. Though it hydrates rapidly, it does not contribute much to the strength of the cement. 5. Magnesia (MgO) The manufacturing process of Portland cement uses magnesia as a raw material in dry process plants. An excess amount of magnesia may make the cement unsound and expansive, but a little amount of it can add strength to the cement. Production of MgO-based cement also causes less CO2 emission. All cement is limited to a content of 6% MgO. 6. Sulphur trioxide Sulfur trioxide in excess amount can make cement unsound. 7. Iron oxide/ Ferric oxide Aside from adding strength and hardness, iron oxide or ferric oxide is mainly responsible for the color of the cement. 8. Alkalis The amounts of potassium oxide (K2O) and sodium oxide (Na2O) determine the alkali content of the cement. Cement containing large amounts of alkali can cause some difficulty in regulating the setting time of cement. Low alkali cement, when used with calcium chloride in concrete, can cause discoloration. In slag-lime cement, ground granulated blast furnace slag is not hydraulic on its own but is "activated" by addition of alkalis. There is an optional limit in total alkali content of 0.60%, calculated by the equation Na2O + 0.658 K2O. 9. Free lime Free lime, which is sometimes present in cement, may cause expansion. 10. Silica fumes Silica fume is added to cement concrete in order to improve a variety of properties, especially compressive strength, abrasion resistance and bond strength. Though setting time is prolonged by the addition of silica fume, it can grant exceptionally high strength. Hence, Portland cement containing 5-20% silica fume is usually produced for Portland cement projects that require high strength. 11. Alumina Cement containing high alumina has the ability to withstand frigid temperatures since alumina is chemical-resistant. It also quickens the setting but weakens the cement. PROGRESS CHECK Note: Progress check will be given as a summative assessment and to be announced by your teacher. REFERENCES Brown, T. L., Jr., H. E., Bursten, B. E., Murphy, C., Woodward, P., Langford, S., Sagatys, D., & George, A. (2013). Chemistry: The central science. Pearson Higher Education AU. Mortimer, Charles E. (1986). Chemistry 6th Edition, National Bookstore, Inc.