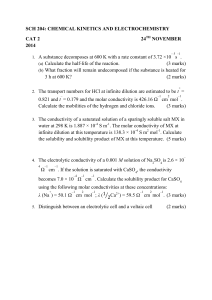
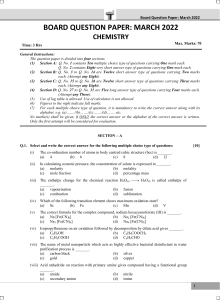
Chapter 8 Open Book Assignment Name: 1. At 25 C, the hydroxide in concentration in normal human blood is 2.5 x 10-7 mol/ L. Calculate the hydrogen ion (H+) concentration and the pH (3 marks) 2. a) Determine the acid-base and conjugate acid-base pairs in the chemical these chemical reactions. (3 marks) b) List the acids from these equations in order from strongest to weakest. (Hint: use page 726-727) (1 mark) 3. Calculate the percent ionization of a 0.050 M solution of an unknown weak acid if the pH of the solution is 2.78. (3 marks) 4. Strychnine is a weak base but a powerful poison. Calculate the pH of a 0.001 mol/L solution when the Kb= 1.0 x 10-6 (5 marks)