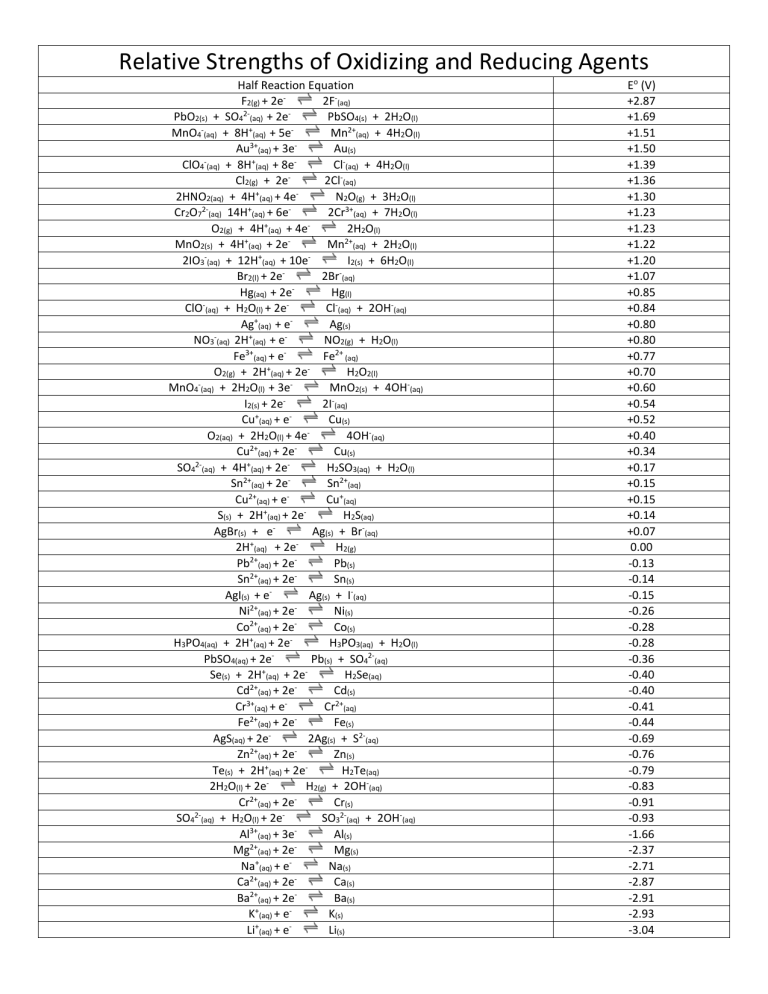
Relative Strengths of Oxidizing and Reducing Agents Half Reaction Equation F2(g) + 2e2F-(aq) 2PbO2(s) + SO4 (aq) + 2e PbSO4(s) + 2H2O(l) MnO4-(aq) + 8H+(aq) + 5eMn2+(aq) + 4H2O(l) 3+ Au (aq) + 3e Au(s) ClO4-(aq) + 8H+(aq) + 8eCl-(aq) + 4H2O(l) Cl2(g) + 2e2Cl-(aq) 2HNO2(aq) + 4H+(aq) + 4eN2O(g) + 3H2O(l) 2+ Cr2O7 (aq) 14H (aq) + 6e 2Cr3+(aq) + 7H2O(l) + O2(g) + 4H (aq) + 4e 2H2O(l) MnO2(s) + 4H+(aq) + 2eMn2+(aq) + 2H2O(l) 2IO3-(aq) + 12H+(aq) + 10eI2(s) + 6H2O(l) Br2(l) + 2e2Br-(aq) Hg(aq) + 2eHg(l) ClO (aq) + H2O(l) + 2eCl-(aq) + 2OH-(aq) Ag+(aq) + eAg(s) NO3 (aq) 2H+(aq) + eNO2(g) + H2O(l) Fe3+(aq) + eFe2+ (aq) O2(g) + 2H+(aq) + 2eH2O2(l) MnO4 (aq) + 2H2O(l) + 3e MnO2(s) + 4OH-(aq) I2(s) + 2e 2I-(aq) + Cu (aq) + e Cu(s) O2(aq) + 2H2O(l) + 4e4OH-(aq) 2+ Cu (aq) + 2e Cu(s) 2+ SO4 (aq) + 4H (aq) + 2e H2SO3(aq) + H2O(l) Sn2+(aq) + 2eSn2+(aq) 2+ Cu (aq) + e Cu+(aq) + S(s) + 2H (aq) + 2e H2S(aq) AgBr(s) + eAg(s) + Br-(aq) 2H+(aq) + 2eH2(g) 2+ Pb (aq) + 2e Pb(s) Sn2+(aq) + 2eSn(s) AgI(s) + eAg(s) + I-(aq) Ni2+(aq) + 2eNi(s) Co2+(aq) + 2eCo(s) H3PO4(aq) + 2H+(aq) + 2eH3PO3(aq) + H2O(l) PbSO4(aq) + 2ePb(s) + SO42-(aq) + Se(s) + 2H (aq) + 2e H2Se(aq) Cd2+(aq) + 2eCd(s) Cr3+(aq) + eCr2+(aq) 2+ Fe (aq) + 2e Fe(s) AgS(aq) + 2e 2Ag(s) + S2-(aq) 2+ Zn (aq) + 2e Zn(s) Te(s) + 2H+(aq) + 2eH2Te(aq) 2H2O(l) + 2eH2(g) + 2OH-(aq) Cr2+(aq) + 2eCr(s) SO42-(aq) + H2O(l) + 2eSO32-(aq) + 2OH-(aq) Al3+(aq) + 3eAl(s) Mg2+(aq) + 2eMg(s) Na+(aq) + eNa(s) Ca2+(aq) + 2eCa(s) Ba2+(aq) + 2eBa(s) K+(aq) + eK(s) Li+(aq) + eLi(s) Eo (V) +2.87 +1.69 +1.51 +1.50 +1.39 +1.36 +1.30 +1.23 +1.23 +1.22 +1.20 +1.07 +0.85 +0.84 +0.80 +0.80 +0.77 +0.70 +0.60 +0.54 +0.52 +0.40 +0.34 +0.17 +0.15 +0.15 +0.14 +0.07 0.00 -0.13 -0.14 -0.15 -0.26 -0.28 -0.28 -0.36 -0.40 -0.40 -0.41 -0.44 -0.69 -0.76 -0.79 -0.83 -0.91 -0.93 -1.66 -2.37 -2.71 -2.87 -2.91 -2.93 -3.04