Abiotic Factors Lab: Measuring Ecosystem Health
advertisement

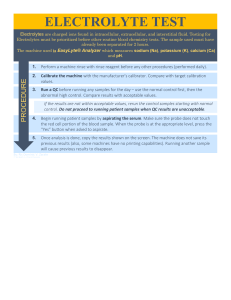
Testing Abiotic Factors Intro Lab Background Information: Throughout the next two years we will be doing many investigations within our environment. In this lab we will learn to use many tools to measure abiotic factors in an ecosystem. As we have studied in class, abiotic factors are all of the nonliving factors in an ecosystems. These factors obviously influence the organisms that live in an ecosystem. Some of these factors include; water, soil, and air. Looking at the state of abiotic factors allows us to look at one aspect of the health of an ecosystem. Throughout this lab you should think about what types of investigations you could carry out using the equipment we are learning about. This will help you prepare for future design labs (IA’s). Aim: Students will learn to use a variety of different tools to measure abiotic factors in an ecosystem. Students will learn to use Logger Pro Software for data collection Students will practice using good lab techniques to gather precise and accurate data Materials Logger Pro software installed on your laptop Go Link Adaptor to plug into your USB port Vernier Temperature Sensor Vernier pH Probe Vernier Dissolved Oxygen (DO) Probe Vernier Turbidity Probe Vernier Conductivity Probe Vernier CO2 Sensor 500 mL beakers Hot water heater Ice Buffer solution pH 4 (See Vernier pH probe instruction guide for making buffer solutions) Buffer solution pH 7 Buffer solution pH 10 4 Household Solutions of varying pH levels 3 Soil samples Digital scale 4 water samples (Gulshan Lake, Sink, Bottled Water, Deionized Water) Safety Considerations: You will be using possibly contaminated soil and water samples so should be sure to wash your hands with soap and water after finishing the lab. Glassware should be handled with care to avoid breakage PROCEDURES: Testing Temperature Air temperature plays an important role in determining they type of organisms that live in an environment. We know from looking at different ecosystems around the world that there are different types of biotic factors that live in warm versus cold climates. This factor, along with amount of yearly precipitation are key factors that determine what biome an ecosystem belongs to. Temperature is an important indicator of the overall quality of a body of water. Since changes in temperature can harm aquatic organisms, it must be monitored carefully. Changes in temperature can also effect other important factors such as the solubility of gases and the photosynthetic rate of aquatic plants. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Open Logger Pro on your laptop Connect GoPro Sensor using USB cable Familiarize yourself with the software Go to Experiment – this is the area where you will set the parameters for your individual experiment. For example, if you want to take data points at specific time intervals, how long you want an experiment to run for, etc. Go to Data Collection Set the probe to collect data every 30 seconds for 3 minutes. Get a 500 mL beaker Fill it with about 300 mL of water from the hot water container Place the probe in the water and click Experiment Start collection Add 4 ice cubes to the beaker. Collect the data Go to File Export As CSV (allows you to open data in Excel) Save this data for use in the PROCESSED DATA SECTION Measuring pH pH is a measure of how acidic or basic a solution is (actually a measure of the number of H+ ions). A neutral solution has a pH value of 7.0. The lower the pH value (closer to 0), the more acidic a solution is. The higher the pH value (closer to 14), the more alkaline (basic) a solution is. Water (H2O) contains both hydrogen ions, H+, and hydroxide ions OH-. To determine the pH of a solution, the relative concentrations of both ions must be measured. Neutral water has an equal concentration of these ions, while acidic solutions have a greater amount of H+ ions and basic (alkaline) solutions have a greater amount of OH- ions. When looking at water quality or soil quality we often look at pH values. If an area is polluted by a lot of acid rain for example we would expect pH values to be lower (more acidic). The pH of soil will affect the types of plants that are able to grow in the soil. Most plants grow best in soil with a pH of 6.5. NOTE: The pH Sensors MUST be stored UPRIGHT and in the Buffer solution container or they will be destroyed! 1. 2. 3. 4. 5. Using the Vernier Labpro equipment and sensors, prepare your computer or calculator for data collection. Remove the storage bottle from the electrode by first unscrewing the lid, then removing the bottle and lid. Thoroughly rinse the lower section of the probe using distilled water. Connect the pH sensor to your lab interface Calibrate the sensor for accurate readings by completing the following steps: a. Use the 2-point calibration option of the Vernier data collection program b. Rinse the tip of the electrode in distilled water. c. Place the electrode into one of the buffer solutions (pH 4) d. When the voltage reading displayed on the computer of calculator screen stabilizes, enter a pH value “4” e. For the next calibration point, rinse the electrode and place it into a second buffer solution (pH 7). f. When the displayed voltage stabilizes enter a pH value of “7” g. For the last calibration point, rinse the electrode and place it into a third buffer solution (pH 10) h. When the displayed voltage stabilizes enter a pH value of “10” i. Rinse the electrode with distilled water and place it in the sample to be measured. 6. Now that the probe has been calibrated you can collect data. 7. Obtain 3 soil samples 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. Record qualitative data about the 3 different soils in your data table. Weigh about 10 grams of soil sample A using the digital scale. Record the exact mass and uncertainty in data table #2. Measure 100 mL of deionized water using the graduated cylinder. Mix the water and soil in a 500 mL beaker Stir the mixture thoroughly using a stirring rod. Repeat steps 10-14 for the other 2 soil samples. Let each sample settle for 5 minutes. While waiting: Measure the pH of at least 4 different household solutions. Leave the sensor in the solution until the values stabilize. Record your data in Data Table 3. When the soil samples have settled, measure the pH of each soil sample. REMEMBER to rinse the sensor with deionized water between each measurement! 20. Rinse the pH sensor with deionized water and return to the buffer solution container for storage. Testing for Dissolved Oxygen Oxygen is a key component to the survival of most aquatic and terrestrial organisms. It is vital in the process of cellular respiration and without sufficient oxygen levels most organisms would not survive. Aquatic organisms get their oxygen from the water in which they live. This oxygen is known as dissolved oxygen. Some organisms require high amounts of dissolved oxygen, while others require lower amounts. Aquatic plant populations and temperature of the water are both contributing factors that influence the amount of dissolved oxygen in a body of water. Dissolved oxygen is affected by both temperature and atmospheric pressure. Dhaka has an elevation of about 60 feet and therefore has an approximate elevation of 760 mmHg. 1. Prepare the Vernier Labpro equipment and calculator. 2. Plug the dissolved oxygen probe into the Vernier interface. 3. Calibrate the sensor for accurate readings by completing the following steps: a. The Optical DO Probe should not need to be calibrated, however if you feel the measurements are off then follow the directions in the probe instruction sheet if needed 4. Choosing Units (mg/L or %) – choose your units on the side of the probe by clicking the switch, record the units you are using on your data table. a. When using this probe you need to decide in your experimental design how you want to measure the DO. (see probe instruction sheet for more details) 5. Rinse the tip of the probe with distilled water and gently blot dry. 6. Place the probe into the sample 1 (Make sure the metal dot near the tip of the probe is submersed for the temperature compensation to work.) 7. Allow probe to sit until it is stabilized (this will take more time if below 15°C or above 30°C) 8. Record data on Data Table 4 9. Repeat steps 5-8 for other 3 solutions. Testing for Turbidity Turbidity is a measure of water’s lack of clarity. Water with high turbidity is cloudy and water with low turbidity is clear. This cloudiness is produced by a large amount of particles present that are reflecting light. When water has very high turbidity the amount of sunlight that can reach lower levels of a body of water is limited. This will limit the amount of plant growth at lower levels, which in turn will affect the amount of dissolved oxygen in the water. 1. Connect the sensor to the interface 2. Start data collection software 3. Calibrate the sensor for accurate readings by completing the following steps: 4. 5. 6. 7. 8. 9. 10. a. Follow instructions in user manual Gently invert the water sample to mix particles that may have settled. (do not shake or air bubbles will affect results) Fill blank cuvette with sample water to the top line. Screw on lid and wipe outside with tissue Hold the cuvette by the lid and place into the sensor aligning the marks. Close lid Record data on Data Table 4(note values will change as particle settle so record as soon as you place in sensor) Clean cuvette with distilled water Repeat steps 4-9 for other three samples Testing Conductivity Conductivity is one of the most common environmental test for aquatic samples. Conductivity is a measure of the ability of water to conduct an electrical current. This is determined byte he amount of dissolved solids (such as salts) in the water. Water containing a lot of ions will have higher conductivity that water with fewer ions. Therefore deionized water will have low conductivity while salt water which has a lot of dissolved salts and therefore many ions, will have high conductivity. Conductivity values allow us to determine if there is a high number of dissolved material in the water. 1. Calibration for the conductivity probe is usually not needed, however when conducting an IA you should follow the calibration procedures in the probe manual. 2. Connect the sensor to the interface. 3. Rinse the tip of the conductivity probe with distilled water. 4. Insert the tip of the probe into sample 1. (make sure electrodes are submerged and that there are no air bubbles) 5. Gently swirl the probe and wait for values to stabilize. (5-10 seconds) 6. Record data on Data Table 4 7. Rinse the probe with distilled water 8. Repeat steps 3-7 for other 3 samples. Testing Carbon Dioxide Levels (modified from Vernier Lab) Carbon dioxide is a colorless and odorless gas. It has many important uses. It is the fizz in soft drinks, beer, and sparkling wines. Carbon dioxide is released by baking powder of yeast to make cake batter rise. It is used in some fire extinguishers. Dry ice is solid carbon dioxide. Carbon dioxide is a product in cellular respiration and a reactant in photosynthesis. But too much of a good thing, like carbon dioxide, can be bad. Carbon dioxide concentration is a surrogate for indoor pollutants that can cause drowsiness, headaches, and lower activity levels. CO2 levels less than 600 ppm are desirable, and levels greater than 1000 ppm are generally considered indicative of inadequate ventilation. Levels of 2,500 ppm or greater can cause headaches. Unfortunately, studies indicate that many classrooms have peak CO2 levels in excess of 2,500 ppm. In the Preliminary Activity, you will gain experience using a CO2 Gas Sensor while monitoring the carbon dioxide concentration in the classroom. 1. 2. 3. 4. Connect the CO2 Gas Sensor and the data-collection interface. Set the data-collection program up to collect data for 180 seconds following your teacher’s instructions. Wait 90 seconds for the sensor to warm up and start data collection. Export your data to an Excel file for data processing. Other Probes Commonly Used in Environmental Science: Colorimeter – measures color changes may be used for photosynthesis experiments or to measure the amounts of phosphates in water (eutrophication experiments) Flow Rate Sensor – used to measure how fast water is flowing Oxygen Gas Sensors – measures oxygen levels in air (not water) RAW DATA: Table 1: Temperature of Water - Organize and format your Temperature Data Using Excel. Table 2: Measuring pH of Soil Samples Soil Sample Mass (___ ± _____) pH Value (±_______) Qualitative (Descriptive) Data A B C Table 3: Measuring pH of 4 known samples Sample Name pH Value (±_______) Qualitative Data Table 4: Water Quality Testing Water Sample Gulshan Lake Water Water from Sink Bottled Water Deionized water DO Level (______ ± _______) Turbidity (______ ± _______) Conductivity (______ ± _______) Qualitative Observations Table 5: Carbon Dioxide Levels in Air - Organize and format your CO2 Data Using Excel. PROCESSED DATA This is where you perform calculations (if needed) and where you display any graphs to make your data easier to look at. Using Excel create graphs for your temperature and carbon dioxide levels. Be sure to change the formatting to make your graph display the data in the “best” format. (Change scale to maximize data trends, give titles, add labeled axes including uncertainties, etc.) DISCUSSION, EVALUATION, AND CONCLUSIONS In this section of the lab you need to summarize your findings. You should discuss any patterns or trends in your data by citing SPECIFIC DATA. You should make comparisons between different trials and compare your data with known results. You should make links between what you saw through your lab and what you know about the science behind it. (What we have studied in class) Your second paragraph should evaluate your lab experiment itself. What were possible sources of error and how could this have affected your data? Did you have any anomalous data points? What could have caused these and how would this affect your end results? Did your qualitative data support your quantitative data? Was your data precise? How do you know? Was your data accurate? How do you know? In your third paragraph you should wrap up your overall experiment. Did your results support or refute your hypothesis? What were the overall patterns or trends? What are the main limitations/weaknesses? How would you improve this experiment if you did this over again? What is the scientific basis for your results? Since most of this does not apply to THIS lab please answer the following questions instead: 1. 2. 3. 4. 5. 6. Why is it important to calibrate your tools before collecting data? What is the difference between accuracy and precision? What information can you use to determine if your data is precise? What information can you use to determine if your data is accurate? What is the formula for determining percentage error? Scooby uses his thermometer and finds the boiling point of ethyl alcohol to be 75o C. He looks in a reference book and finds that the actual boiling point of ethyl alcohol is 78oC. What is his percent error? 7. You perform 3 trials of an experiment, which produces a new compound. You measure the mass of your product to be 0.05, 0.83, and 1.04 g. Find the average mass of your compounds. The experiment should have produced 0.76 g. What is your percent error? 8. Using one of the tools we learned about in this lab. Think of an investigation you could perform to look at one effect of an abiotic factor on an ecosystem. What research question could you investigate? NOTE: This lab contains A LOT of useful information. If I were you I would make sure to keep this in a SAFE PLACE for FUTURE REFERENCE!