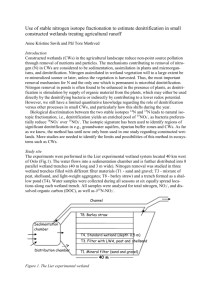
Science of the Total Environment Research progress in solid carbon source–based denitrification technologies for different target water bodies --Manuscript Draft-Manuscript Number: STOTEN-D-20-19231R1 Article Type: Review Article Keywords: solid carbon source, heterotrophic denitrification, nitrogen pollution, nitrogen removal, water treatment Corresponding Author: Shiyang Li, Ph.D. Shanghai University School of Environmental and Chemical Engineering Shanghai, CHINA First Author: Feifan Zhang Order of Authors: Feifan Zhang Chengjin Ma Xiangfeng Huang, Ph.D. Jia Liu, Ph.D. Lijun Lu, Ph.D. Kaiming Peng, Ph.D. Shiyang Li, Ph.D. Abstract: Nitrogen pollution in water bodies is a serious environmental issue which is commonly treated by various methods such as heterotrophic denitrification. In particular, solid carbon source (SCS)–based denitrification has attracted widespread research interest due to its gradual carbon release, ease of management, and long-term operation. This paper reviews the types and properties of SCSs for different target water bodies. While both natural (wheat straw, wood chips, and fruit shells) and synthetic (polybutylene succinate, polycaprolactone, polylactic acid, and polyhydroxyalkanoates) SCSs are commonly used, it is observed that the denitrification performance of the synthetic sources is generally superior. SCSs has been used in the treatment of wastewater (including aquaculture wastewater), agricultural subsurface drainage, surface water, and groundwater; however, the key research aspects related to SCSs differ markedly based on the target waterbody. These key research aspects include nitrogen pollutant removal rate and byproduct accumulation (ordinary wastewater); water quality parameters and aquatic product yield (recirculating aquaculture systems); temperature and hydraulic retention time (agricultural subsurface drainage); the influence of dissolved oxygen (surface waters); and nitrate-nitrogen load, HRT, and carbon source dosage on denitrification rate (groundwater). It is concluded that SCS-based denitrification is a promising technique for the effective elimination of nitrate-nitrogen pollution in water bodies. Response to Reviewers: Reviewer #1:According to the authors, "Based on our meta-analysis we identify and classify the denitrification principles of synthetic SCSs, commonly used SCSs, influencing factors, and effluent parameters." However, I did not see any meta-analysis of the existing results in the paper. More importantly, the authors mainly summarized the findings of past studies and did not provide in-depth analyses of these results. Especially in section 3, I expected the authors to give a critical analysis of the findings of previous studies on the application of SCSs in different target water bodies, but in my view that section of the paper merely presented a summary of the results of the past studies. Therefore, I do not see it to be of much interest to the STOTEN readership. Answer: Thanks a lot for the suggestion. Detailed descriptions of meta-analysis on synthetic SCS-based denitrification and further discussion on the effect of different SCSs and reaction conditions on the denitrification efficiency were supplemented in the revised Powered by Editorial Manager® and ProduXion Manager® from Aries Systems Corporation manuscript as a section of ‘ Meta-analysis on synthetic SCS-based denitrification’. During the meta-analysis, process of data compilation, determination of target factors and application of software were detailedly introduced in the revised manuscript, and the results of nitrate removal rate effect size and its standard deviation were expressed visually in the form of five forest plots, which were also supplemented as Figure 2. The actual data on mean removal rate, SD, number of studies and the classification of each factors are listed in Table S1 in Supplementary Information. Based on the results from meta-analysis, the effect of carbon source type, carbon source species, influent N concentration, HRT and water temperature on nitrate removal rates were discussed respectively, and finally provide scientific supports for the future applications of synthetic SCS-based denitrification. Due to the supplements of a novel section, some adjustments has been made in Introduction and Conclusion parts. In the Introduction: “In this paper, we provide an overview both lab and field studies of the types and operating principles of commonly used SCSs based on a review in this field. We identify and classify the denitrification principles of synthetic SCSs, commonly used SCSs, influencing factors, and effluent parameters. Meta-analysis was further applied for better understanding of the effect of different SCSs and reaction conditions on the denitrification efficiency. Finally, we summarize the limitations governing the use of SCSs to provide a scientific basis for the future development of SCS-based denitrification techniques.” In the Conclusion “Solid-phase carbon sources have good application prospects for solid phase nitrification. Research with respect to wastewater treatment has mainly been focused on the removal efficiency of nitrogen pollutants and DOC accumulation in the effluent, while studies on recirculating aquaculture systems have focused on product yield and water quality parameters. Agricultural subsurface drainage system research was extensive, and focused on natural SCSs and the influence of temperature and HRT on denitrification efficiency. The primary aspect of surface water research was the influence of DO on denitrification efficiency, while studies on groundwater were mainly focused on the influence of nitrate-nitrogen load, HRT, and carbon source dosage on denitrification efficiency. The optimization of operation parameters (especially HRT), the hybrid application of synthetic carbon sources and the further design of low temperature-tolerated reactors are worthy of continued study.” Reviewer #3:I especially notice one component that is missing from the review. That is the scientific basis of all prominent technologies mentioned for the denitrification. With the detailed underlying processes and pathways and then highlighting differences in the process for which one method is better than the other will certainly enrich this review. Answer: Thanks a lot for the suggestion. For better illustration of the scientific basis of SCSbased denitrification and comparing the differences of different SCSs during denitrification process, a section of ‘Comparison of raw and synthetic SCSs’ was supplemented in the revised manuscript. Since raw and synthetic SCSs are the commonly used carbon sources for biological denitrification, the comparison between the two types of SCSs were extensively discussed in this section. We analyzed their differences in material sources, hydrolytic ability and hydrolysate, as well as denitrification performance. Besides, their adaptive applications in different target water bodies were also summarized and discussed. Due to the supplement of new contents, the title for the second section has been adapted as ‘Types, properties and utilization of SCSs’ for better summarization. Powered by Editorial Manager® and ProduXion Manager® from Aries Systems Corporation Cover letter [Feb 25, 2020] Jay Gan Editor in Chief Science of the Total Environment Dear Editor: I wish to submit a revised version of our manuscript for publication in Science of the Total Environment, titled “Research progress in solid carbon source–based denitrification technologies for different target water bodies.” The paper was coauthored by Feifan Zhang, Chengjin Ma, Xiangfeng Huang, Jia Liu, Lijun Lu, Kaiming Peng. We would like to thank you for giving us the opportunity to submit a revised version of our manuscript and the reviewers for their helpful suggestions and comments. We have replied to each comment in detail and revised the manuscript accordingly. We specifically cleared some unclarities about our collection efficiency, which was a concern of both reviewers. For a detailed point-by-point response, please refer to the attached response letter. This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these. There are no conflicts of interest to declare. Thank you for your consideration. I look forward to hearing from you. Sincerely, Shiyang Li College of Environmental Science and Engineering, Tongji University, Shanghai 200092, People’s Republic of China. Tel: +86 021 65982399 Email: lishiyang@tongji.edu.cn *Title Page 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 Solid carbon source–based denitrification technologies for different target water bodies: A review Chengjin Ma a, Xiangfeng Huang a, Jia Liu a, Lijun Lu a, Kaiming Peng a, Shiyang Li a a College of Environmental Science and Engineering, State Key Laboratory of Pollution Control and Resource Reuse, Ministry of Education Key Laboratory of Yangtze River Water Environment, Tongji University, Shanghai 200092, People’s Republic of China Correspondence information: Shiyang Li, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, People’s Republic of China. Tel: +86 021 65982399. Email: lishiyang@tongji.edu.cn Reviewer #1: According to the authors, "Based on our meta-analysis we identify and classify the denitrification principles of synthetic SCSs, commonly used SCSs, influencing factors, and effluent parameters." However, I did not see any meta-analysis of the existing results in the paper. More importantly, the authors mainly summarized the findings of past studies and did not provide in-depth analyses of these results. Especially in section 3, I expected the authors to give a critical analysis of the findings of previous studies on the application of SCSs in different target water bodies, but in my view that section of the paper merely presented a summary of the results of the past studies. Therefore, I do not see it to be of much interest to the STOTEN readership. Answer: Thanks a lot for the suggestion. Detailed descriptions of meta-analysis on synthetic SCS-based denitrification and further discussion on the effect of different SCSs and reaction conditions on the denitrification efficiency were supplemented in the revised manuscript as a section of ‘ Meta-analysis on synthetic SCS-based denitrification’. During the meta-analysis, process of data compilation, determination of target factors and application of software were detailedly introduced in the revised manuscript, and the results of nitrate removal rate effect size and its standard deviation were expressed visually in the form of five forest plots, which were also supplemented as Figure 2. The actual data on mean removal rate, SD, number of studies and the classification of each factors are listed in Table S1 in Supplementary Information. Based on the results from meta-analysis, the effect of carbon source type, carbon source species, influent N concentration, HRT and water temperature on nitrate removal rates were discussed respectively, and finally provide scientific supports for the future applications of synthetic SCS-based denitrification. Due to the supplements of a novel section, some adjustments has been made in Introduction and Conclusion parts. In the Introduction: “In this paper, we provide an overview both lab and field studies of the types and operating principles of commonly used SCSs based on a review in this field. We identify and classify the denitrification principles of synthetic SCSs, commonly used SCSs, influencing factors, and effluent parameters. Meta-analysis was further applied for better understanding of the effect of different SCSs and reaction conditions on the denitrification efficiency. Finally, we summarize the limitations governing the use of SCSs to provide a scientific basis for the future development of SCS-based denitrification techniques.” In the Conclusion “Solid-phase carbon sources have good application prospects for solid phase nitrification. Research with respect to wastewater treatment has mainly been focused on the removal efficiency of nitrogen pollutants and DOC accumulation in the effluent, while studies on recirculating aquaculture systems have focused on product yield and water quality parameters. Agricultural subsurface drainage system research was extensive, and focused on natural SCSs and the influence of temperature and HRT on denitrification efficiency. The primary aspect of surface water research was the influence of DO on denitrification efficiency, while studies on groundwater were mainly focused on the influence of nitrate-nitrogen load, HRT, and carbon source dosage on denitrification efficiency. The optimization of operation parameters (especially HRT), the hybrid application of synthetic carbon sources and the further design of low temperature-tolerated reactors are worthy of continued study.” Reviewer #3: I especially notice one component that is missing from the review. That is the scientific basis of all prominent technologies mentioned for the denitrification. With the detailed underlying processes and pathways and then highlighting differences in the process for which one method is better than the other will certainly enrich this review. Answer: Thanks a lot for the suggestion. For better illustration of the scientific basis of SCS-based denitrification and comparing the differences of different SCSs during denitrification process, a section of ‘Comparison of raw and synthetic SCSs’ was supplemented in the revised manuscript. Since raw and synthetic SCSs are the commonly used carbon sources for biological denitrification, the comparison between the two types of SCSs were extensively discussed in this section. We analyzed their differences in material sources, hydrolytic ability and hydrolysate, as well as denitrification performance. Besides, their adaptive applications in different target water bodies were also summarized and discussed. Due to the supplement of new contents, the title for the second section has been adapted as ‘Types, properties and utilization of SCSs’ for better summarization. Research progress in solid carbon source–based denitrification technologies for different target water bodies Feifan Zhang a, Chengjin Ma a, Xiangfeng Huang a, Jia Liu a, Lijun Lu a, Kaiming Peng a, Shiyang Li a a College of Environmental Science and Engineering, State Key Laboratory of Pollution Control and Resource Reuse, Ministry of Education Key Laboratory of Yangtze River Water Environment, Tongji University, Shanghai 200092, People’s Republic of China Correspondence information: Shiyang Li, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, People’s Republic of China. Tel: +86 021 65982399. Email: lishiyang@tongji.edu.cn Abstract: Nitrogen pollution in water bodies is a serious environmental issue which is commonly treated by various methods such as heterotrophic denitrification. In particular, solid carbon source (SCS)–based denitrification has attracted widespread research interest due to its gradual carbon release, ease of management, and long-term operation. This paper reviews the types and properties of SCSs for different target water bodies. While both natural (wheat straw, wood chips, and fruit shells) and synthetic (polybutylene succinate, polycaprolactone, polylactic acid, and polyhydroxyalkanoates) SCSs are commonly used, it is observed that the denitrification performance of the synthetic sources is generally superior. SCSs has been used in the treatment of wastewater (including aquaculture wastewater), agricultural subsurface drainage, surface water, and groundwater; however, the key research aspects related to SCSs differ markedly based on the target waterbody. These key research aspects include nitrogen pollutant removal rate and byproduct accumulation (ordinary wastewater); water quality parameters and aquatic product yield (recirculating aquaculture systems); temperature and hydraulic retention time (agricultural subsurface drainage); the influence of dissolved oxygen (surface waters); and nitrate-nitrogen load, HRT, and carbon source dosage on denitrification rate (groundwater). It is concluded that SCSbased denitrification is a promising technique for the effective elimination of nitrate-nitrogen pollution in water bodies. Keywords: solid carbon source, heterotrophic denitrification, nitrogen pollution, nitrogen removal, water treatment 1 Introduction A fast increase in human population and rapid developments in the industrial and agricultural sectors have resulted in nitrogen pollution–induced eutrophication of water bodies (Jafari et al., 2015, Liu et al., 2019). In natural water bodies, nitrogen existing in forms other than nitrate-nitrogen is gradually converted to nitrate-nitrogen by microorganisms, resulting in an increasingly severe accumulation of nitrate-nitrogen in water bodies (Canfield et al., 2010, Zhang et al., 2014). Water bodies containing excessive nitrate-nitrogen may cause damage to crop root systems and impact crop yields when used as water sources for agricultural irrigation (Steidl et al., 2019). Furthermore, drinking from polluted water bodies may cause toxic effects or even death in wildlife, and excessive nitrate-nitrogen intake in humans can result in methemoglobinemia (blue baby syndrome) or may cause toxic effects or even death in wildlife in extreme cases (Li et al., 2017). Studies have revealed that nitrogen pollution in groundwater exists in approximately 110 countries and has caused safety issues concerning drinking water globally (Chen et al., 2013, Feng et al., 2020b). In response to existing nitrogen pollution issues, many physicochemical and bioecological methods have been utilized for the removal of excess nitrate-nitrogen in water. Owing to the current technological progress, the application of carbon sources under anoxic conditions to achieve biological nitrogen removal through denitrification (Cheng et al., 2020, Zhang et al., 2017) has emerged as the mainstream method employed in water treatment. Treatment processes in traditional wastewater treatment plants usually involve the addition of external carbon sources to promote microbial denitrification. Commonly used external carbon sources mainly consist of water-soluble low-molecular-weight (LMW) organic compounds such as methanol, acetates, and LMW sugars (Feng et al., 2013). However, the addition of dissolved carbon sources to natural water bodies increases the organic pollution load and may even cause secondary pollution if the calculated dosage amounts are unreasonable. Furthermore, the need for dosing facilities is also a limiting factor in the widespread use of dissolved carbon sources. Conversely, solid carbon sources (SCSs) have attracted a considerable research interest due to their appropriate carbon release rates, favorable conditions for microbial biofilm growth, long carbon release duration, ease of management, and long-term operation(Yang et al., 2020b, Zhang et al., 2020). Currently, the most commonly used SCSs can be classified into two major categories, i.e, natural cellulosic materials and synthesized biodegradable polymers (BDPs). Target water bodies for SCS-based denitrification include wastewater (including aquaculture wastewater), agricultural subsurface drainage, surface water, and groundwater. To date, studies regarding the application of SCSs to different target water bodies under specific conditions and operating requirements have investigated the types and properties of commonly used carbon sources, process characteristics, influencing factors, and effluent parameters. In this paper, we provide an overview both lab and field studies of the types and operating principles of commonly used SCSs based on a review in this field. We identify and classify the denitrification principles of synthetic SCSs, commonly used SCSs, influencing factors, and effluent parameters. Meta-analysis was further applied for better understanding of the effect of different SCSs and reaction conditions on the denitrification efficiency. Finally, we summarize the limitations governing the use of SCSs to provide a scientific basis for the future development of SCS-based denitrification techniques. 2 Types, properties and utilization of SCSs 2.1 Commonly used SCSs Commonly used SCSs can be classified into three major categories: natural (cellulosic), synthetic (polymeric), and other SCSs (e.g., acidogenic liquids from food waste, hydrolyzed sludge and other reprocessed organic materials) (Guo et al., 2017, Zhang et al., 2016a). Due to the scarcity of relevant existing literature and applications, other SCSs have not been discussed in this paper. Most early applications of SCSs involved the use of natural carbon sources, including cellulosic agricultural and forestry wastes, such as corn cobs, corn stover, wheat straw, cardboard fibers, leaf litter, tree bark, wood chips, and fruit kernels, which were reported in previous studies (Chang et al., 2016, Christianson et al., 2012, Chun et al., 2009, Gomez et al., 2000). Natural SCSs have several advantages such as low cost and ease of acquisition. Because they consist primarily of agricultural and forestry wastes, they are commonly used as filling materials in denitrification located near the outflow of agricultural subsurface drainage systems. However, the wider application of natural SCSs in denitrification is limited by unstable carbon release rates, slow denitrification rates, excessive release of dissolved organic carbon (DOC), and increased color intensity in the effluent. Therefore, while the denitrification efficiencies of natural SCSs were reported more frequently in earlier studies, more recent literature has focused on the simultaneous removal of total nitrogen (TN) and nitrate-nitrogen (Si et al., 2018), aerobic denitrification (Cheng et al., 2020), characteristics of leached pollutants (Abusallout and Hua, 2017, Jia et al., 2019), and functional microbial communities (Hu et al., 2019). In recent years, the use of BDPs as synthetic SCSs has attracted considerable research attention. BDPs only decompose under the action of extracellular enzymes secreted by specific microorganisms; hence, they can avoid or mitigate many of the aforementioned problems associated with natural SCSs. BDPs refer to a class of high-molecular-weight (HMW) materials that are degraded or enzymatically hydrolyzed under biological action to generate LMW compounds which can be utilized by organisms. In addition to serving as carbon sources, BDPs as synthetic SCSs also act as carriers for the growth of denitrifying microorganisms (Zhang et al., 2016c; d) and have been used in the denitrification of water bodies since the 1990s. In 1992, Müller et al. reported the use of polyhydroxybutyrate (PHB) granules as an SCS for denitrification through the construction of a laboratory-scale up flow fixed-bed reactor (Muller et al., 1992). They found that the denitrification rate at 10 ºC was 11 mg·(L·h)-1, and cells co-immobilized with the PHB granules exhibited a higher denitrification rate compared to suspended cells. Currently, only a few types of synthetic SCSs are commonly applied to the denitrification of wastewater, including polylactic acid (PLA) (Fan et al., 2012), polycaprolactone (PCL) (Chu and Wang, 2011a; b, Li et al., 2016, Wu et al., 2013), polybutylene succinate (PBS) (Wang and Chu, 2016), and polyhydroxyalkanoates (PHAs) (Lopardo and Urakawa, 2019). Table 1 illustrates a summary of the denitrification efficiencies reported in a number of studies on SCSs. Table 1. Summary of denitrification performance of different solid carbon source (SCSs) reported in previous literature 2.2 Basic principles of synthetic SCS-based denitrification 2.2.1 Synthetic SCSs Utilization Anoxic biological denitrification usually takes place under the action of microorganisms and fungus. It involves biological redox reactions in which organic carbon sources and nitrates serve as electron donors and acceptors, respectively. This results in the reduction of nitrate to nitrogen, which is subsequently removed from the water body through the denitrification process. The overall process consists of the following steps: 𝑁𝑂3− → 𝑁𝑂2− → 𝑁𝑂 → 𝑁2 𝑂 → 𝑁2 However, only soluble biodegradable carbon source such as acetic acid, formic acid, and methanol can be directly utilized by denitrifying microorganisms during biological denitrification, whereas SCSs must be converted into LMW compounds prior to utilization by microorganisms. The utilization of BDPs as carbon sources and bacterial carriers for SCS based denitrification occurs under microbial action, with SCS biodegradation enabling the growth and metabolism of microorganisms attached to the surfaces of SCSs and the . Polymer degradation can be characterized as a process that results in the breakage of a large and complex molecule into smaller molecules (dos Santos et al., 2018). First, biofilms are formed through the attachment and growth of microorganisms on polymer surfaces; then, polymer chains are cleaved by extracellular enzymes, leading to the hydrolysis of the polymers into soluble LMW compounds (Hocking et al., 1996, Shah et al., 2008). Subsequently, LMW compounds enter functional microorganisms through semipermeable membranes and are utilized as carbon sources and electron donors. In short, the utilization of BDPs involves hydrolysis and denitrification, with the former being the rate- determining process (Takahashi et al., 2011). Figure 1. Schematic of the utilization of biodegradable polymers (BDPs) as carbon sources by denitrifying microorganisms Microbial degradation has various impacts on SCSs, including changes in chemical structure (Lucas et al., 2008), significant reductions in the polymer molecular weight, increased surface roughness, formation of perforations and pits, and mechanical deformation due to structural dissolution and breakdown. In turn, these impacts change the carbon release rate of the material. Microbial degradation also leads to a decrease in the crystalline phase content and a corresponding increase in readily hydrolysable amorphous content, resulting in hydrophilicity changes. 2.2.2 Basic mechanisms of synthetic SCS-based denitrification Müller et al. had reported the use of PHB (molecular formula: [C4H6O2]n) as a synthetic SCS (Muller et al., 1992). And PHB based denitrification reaction with nitrate ions as electron acceptors is as follows: 5[𝐶4𝐻6 𝑂2 ] + 18𝑁𝑂3 − → 9𝑁2 + 18𝐻𝐶𝑂3 − + 2𝐶𝑂2 + 6𝐻2𝑂 (1) According to Boley et al., by assuming a yield coefficient (Yx/s) of 0.45 g biomass/g PHB, the summarized denitrification equation including biomass formation when PHB is used as the carbon source can be expressed as (Boley et al., 2000): 0.494[𝐶4 𝐻6𝑂2 ] + 𝑁𝑂3 − → 0.415𝑁2 + 𝐻𝐶𝑂3 − + 0.130𝐶𝑂2 + 0.169[𝐶5 𝐻7𝑂2 𝑁] (2) + 0.390𝐻2 𝑂 where C5H7O2N is the molecular formula of the microbial cells. Because the molecular formulae of commonly used synthetic SCSs (PCL, PBS, PLA, and PHAs) can be represented as CxHyOz, the basic denitrification reaction for synthetic SCSs is as follows: 5[𝐶𝑥 𝐻𝑦 𝑂𝑧 ] + (4𝑥 + 𝑦 − 2𝑧)𝑁𝑂3 − → (2𝑥 + 𝑦 − − 𝑧)𝑁2 + (4𝑥 + 𝑦 − 2𝑧)𝐻𝐶𝑂3 + (𝑥 − 𝑦 + 2𝑧)𝐶𝑂2 2 (3) + (2𝑦 − 2𝑥 + 𝑧)𝐻2𝑂 Using Equation (3), the calculated mass of PHB required to reduce the unit mass of nitratenitrogen is 2.92 g PHB/g NO3--N when biomass formation is excluded and 3.03 g PHB/g NO3--N when biomass formation is considered. Based on theoretical calculations, the masses of glucose, methanol, and ethanol required to reduce the unit mass of nitrate-nitrogen when biomass formation is excluded are 2.68, 1.90, and 1.37 g/g NO3--N, respectively. When biomass formation is considered, the required masses of methanol and ethanol are 2.47 and 2.01 g/g NO3--N, respectively (Mateju et al., 1992). 2.3 Comparison of raw and synthetic SCSs Compared with liquid carbon sources, SCSs can be used as biofilm carriers for microorganisms, which are more convenient for transportation, operation and storage (Boley et al., 2000). In previous studies, most solid carbon sources can release organic matter needed in denitrification systems, especially in low C/N wastewater treatment. In terms of material sources, raw and synthetic SCSs come from multiple sources. Cellulosic agricultural and forestry wastes are the main components of raw SCSs. Synthetic SCSs are mainly produced by petrochemical engineering, while some studies focus on the utilization of endogenous accumulation of PHAs by microorganisms (He et al., 2018, Wu et al., 2021). With the development of material science, more and more new materials are used in sewage treatment. BDPs with excellent biocompatibility, biodegradability and non-toxicity, such as PCL and PBS, are ideal materials for environmental protection (Wu et al., 2012). The hydrolytic ability of carbon sources is regarded as a key factor of denitrification (Hang et al., 2016). Denitrification rate is inhibited when available carbon from carbon source is insufficient, while an over-release of carbon may on the contrary, cause carbon loss and organic pollution (Healy et al., 2012). Regarding the characteristics of carbon release, first-order kinetics can basically describe the carbon release process of all SCSs. Raw SCSs can release more organic matter, and synthetic SCSs have an advantage in the sustainability of carbon release. In order to take advantage of the advantages of both kinds of SCSs in terms of carbon release, some carbon sources mixed with raw and synthetic SCSs have been prepared (Jiang et al., 2020, Liu et al., 2018b, Xiong et al., 2019) . The hydrolysate of raw and synthetic SCSs are quite different. Raw SCSs often contain multiple components. Taking lignocellulosic materials as an example, they are mainly comprised of lignin, cellulose and hemicellulose. Among them, cellulose can be easily used by microorganisms and hydrolyzed into glucose, hemicellulose can also be used after enzymatic hydrolysis into small molecular organic matter, and lignin is difficult to be degraded (Forrest et al., 2010, Zhong et al., 2020). It has been reported that the denitrification efficiency of woody biomass is lower than that of herbaceous biomass due to its lignin content and natural structure in woody biomass (Kim et al., 2016). The hydrolysate of the synthetic SCSs is related to its own structure, and its degradation involves the joint effect of several processes. Taking PHB as an example, PHB can directly undergo abiotic hydrolysis in water because it contains -COO- group as a polyester. When PHB is used as SCS in a biological denitrification system, biodegradation of PHB also plays a significant role. Enzymes in the intracellular and extracellular matrix can disrupt long-chain polymer chains and hydrolyze oligomers, and these oligomers will be further hydrolyzed into polymer monomers after a short period of time (dos Santos et al., 2018, Kessler et al., 2014). Water temperature, pH value, microbial community and the supplement of nutrients will all affect the degradation process of SCSs. In terms of denitrification, both SCSs can promote denitrification in low C/N wastewater treatment. Due to the difference in carbon release patterns between the two types of carbon sources, and considering the sustainability of carbon release, synthetic SCSs are ideal slow-release carbon sources for low TN water treatment (such as groundwater and drinking water), raw SCSs may be a more ideal carbon source to enhance the denitrification of secondary wastewater (Chu and Wang, 2013, Xiong et al., 2020). 3 Application of SCSs in different target water bodies Currently, the target water bodies for SCS-based denitrification technologies include wastewater (Duan et al., 2016, Xu et al., 2018a, Yang et al., 2020b) (including aquaculture wastewater (Gutierrez-Wing et al., 2012, Zhang et al., 2016b)), agricultural subsurface drainage, (Christianson et al., 2016, Chun et al., 2010), surface water(Feng et al., 2019), and groundwater (Zhang et al., 2018). 3.1 Wastewater Studies on the application of SCSs to wastewater denitrification can be classified into two main categories; (1) advanced treatment of ordinary wastewater or wastewater treatment plant effluent and (2) purification of nitrogen pollutants in recirculating aquaculture systems. 3.1.1 Treatment of ordinary wastewater For wastewater with characteristically low C/N ratios, nitrogen removal is often achieved via dosing with external carbon sources. Generally, SCSs are used as filling materials and microbial carriers in fixed-bed reactors for the purification of wastewater by denitrification. In a study by Rout et al. (Rout et al., 2017), organic solid waste substances were used as carbon sources to investigate the influence of experimental parameters, such as influent nitrate concentration, hydraulic retention time (HRT), and bed depth, on denitrification efficiency. The found that a low HRT reduced nitrate removal efficiency, increased nitrite accumulation, and decreased effluent chemical oxygen demand (COD). When the influent nitrate concentration was 70, 50, and 30 mg/L, the effluent nitrate concentration could be maintained at < 10 mg/L for 31, 39, and 49 days, respectively, with the denitrification process following first-order reaction kinetics. Sun et al. (Sun et al., 2019a) utilized alkali-pretreated corn cobs as solid carbon sources and biofilm carriers for the removal of nitrates and refractory organic pollutants from coking wastewater. They found that the treatment process could concurrently achieve the stable removal of over 90 % of residual nitrate and the degradation of typical refractory organic matter. In another study, Duan et al. compared the performances of PBS, PHBV, and PCL as carbon sources for the treatment of nitrified swine wastewater (Duan et al., 2016). They found that the denitrification reaction time was shortest when PCL was used, with the nitrate removal rate exceeding 95 % after 20 days of cultivation, and total organic carbon (TOC) and NH4+-N were absent in the effluent. Xu et al. constructed a packed-bed bioreactor using a PHBV/PLA blend as a carbon source and biofilm carrier for the removal of ammonia-nitrogen, nitrite-nitrogen, and nitrate-nitrogen from the effluent of a secondary settling tank in an activated sludge wastewater plant (Xu et al., 2019b). They found that the nitrogen removal system effectively removed all three nitrogen pollutants. Furthermore, the nitrogen removal efficiency was influenced by temperature (the denitrification rate at 30 ºC was five times greater than at 10 ºC); however, higher temperatures also promoted TOC accumulation. Sun et al. (Sun et al., 2020) also used a PHBV/PLA blend for the purification of sewage treatment plant effluent, and achieved removal efficiency of 98.1 ± 2.9, 87.2 ± 6.8, and 89.3 ± 6.3 % for NH4+-N, NO3—N, and TN, respectively. These results indicate that the reactor system was capable of simultaneous nitrification and denitrification under appropriate aeration conditions. Previous studies on SCS-based wastewater treatment utilized both natural and synthetic SCSs and mainly focused on the impact of the type of carbon source, temperature, HRT, and pH on treatment efficiency. In light of the objectives of wastewater treatment, a significant number of relevant studies have also examined nitrite and ammonia-nitrogen accumulation and effluent TOC, whereas other studies have investigated changes in microbial communities and the abundance of functional genes. 3.1.2 Recirculating aquaculture systems Recirculating aquaculture systems have emerged as a novel aquaculture technology that involve the treatment of aquaculture wastewater and subsequent recycling and reuse of the treated water. The removal of nitrate-nitrogen represents a key step in the wastewater purification process (Bao et al., 2019, Podduturi et al., 2020). In a study by Luo et al. (Luo et al., 2019), the denitrification performance and bacterial properties of recirculating aquaculture systems using PCL and PHBV as SCSs were compared over a 102 day period. They found that the denitrification rates achieved with PCL and PHBV under influent nitrate-nitrogen concentrations of 81.1–132.75 mg/L and an influent flow rate of 1 L/h were 0.27 and 0.19 g·(L·d)-1, respectively. For the removal of the same mass of nitrate-nitrogen, the mass of PCL consumed was significantly lower than the mass of PHBV, and the effluent nitrate-nitrogen and ammonia-nitrogen concentrations achieved using PCL were also lower. Deng et al. investigated the influence of operating conditions such as dissolved oxygen (DO) concentration and salinity on nitrogen removal performance and microbial communities in a recirculating aquaculture system that utilized PBS as the carbon source (Deng et al., 2017). They found that salinity decreased the number and diversity of operational taxonomic units, while DO had no significant influence on the microbial community. Zhu et al. constructed a denitrification bioreactor using PBS as the carbon source and compared the denitrification performance using real and synthetic recirculating aquaculture system wastewater to determine the influence of salinity and nitrate concentration on heterotrophic denitrification (Zhu et al., 2015). They found that the nitrate volumetric removal rate increased with influent nitrate loading. Conversely, salinity had little influence on nitrate removal (an increase of salinity from 0‰ to 25‰ led to an increase of denitrification rate from 0.53 to 0.66 kg NO3--N·(m3·d)-1); however, it did increase the likelihood of excessive DOC and ammonia-nitrogen accumulation in the effluent. Li et al. prepared a novel beadshaped SCS using semen litchi (SL), poly (vinyl alcohol) (PVA), and sodium alginate (SA) as raw materials (Li et al., 2019). They found that the denitrification rate was up to 243.5 ± 7.08 mg N·(L·d)-1 when the beads were used in the SCS based denitrification of mariculture wastewater. Zhang et al. (Zhang et al., 2016b) introduced PHB as a denitrification carbon source into an aquaculture system in which 120 tilapias were reared. After 120 days of culture without water exchange, it was found that the nitrate-nitrogen concentration of the system was maintained at a certain level, thereby effectively avoiding the toxic effects of nitrate-nitrogen on aquatic animals. Existing studies have indicated that the SCSs used in most recirculating aquaculture systems mainly consist of synthetic SCSs and other novel SCSs. This is because aquaculture systems are sensitive to effluent quality, requiring the use of synthetic carbon sources which can provide stable carbon release rates, less DOC accumulation, and lower effluent color intensity. The main aspects of interest in relevant studies were the conventional performance indicators of denitrification, including nitrate concentration, nitrite, and ammonia accumulation; TOC; and microbial community composition. In certain studies, quality changes in aquatic products (e.g., fishes) reared in systems with and without denitrification were compared; however, significant differences were not observed between the experimental and control groups (Boley and Müller, 2005). 3.2 Agricultural subsurface drainage systems Agricultural subsurface drainage systems are a commonly used agricultural drainage method in which excess groundwater and surface water is removed through underground (subsurface) drainage pipes (Schipper et al., 2010). The controlled agricultural drainage enables the elimination of waterlogging and control of groundwater levels, which are beneficial to the prevention of soil swamping and salinization, creating favorable conditions for agricultural production. In most existing subsurface drainage systems in China, water is directly discharged into nearby water bodies, and has a deleterious impact on the ecological environment (Chun et al., 2009). Consequently, the installation of denitrification bioreactors prior to drainage discharge has become widely accepted. The use of corn cobs, corn stover, wheat straw, cardboard fibers, leaf litter composts, tree bark, wood chips, and almond shells as carbon sources have been reported in previous studies (Christianson et al., 2016, Chun et al., 2009). Camilo et al. (Krause Camilo, 2016) constructed a horizontal flow reactor filled with wheat straw and pine bark mulch for the removal of nitratenitrogen and the herbicide agent atrazine (ATR) from subsurface drainage water. At 21 ºC and a HRT of 0.43 d, the removal rates of nitrate-nitrogen and ATR were 30 g N·(m·d)−1 and 22 mg ATR·(m·d)−1, respectively. David et al. (David et al., 2016) performed a three-year evaluation of two wood chip bioreactors and found that nitrate-nitrogen removal requirements could be satisfied during year one and the early part of year two due to the adequate release of soluble carbon. However, as operating time increased, temperature became the primary limiting factor of the nitrate-nitrogen removal rate. In another study, Li et al. (He et al., 2018) utilized wood chips and fly ash in tandem for the simultaneous removal of nitrate-nitrogen and phosphate pollution from subsurface drainage water. They found that the nitrate-nitrogen removal efficiency changed significantly with HRT. However, changes in phosphate removal efficiency with HRT were not significant, and orthophosphate adsorption by fly ash was far less than the saturated capacity determined from a previous study. The use of biomass denitrification beds for the purification of agricultural subsurface drainage water represents the main practical application of SCS-based denitrification technologies. Research in this field is also relatively well-established and highly relevant to practical applications. SCSbased denitrification beds located at the end of agricultural subsurface drainage systems mainly utilize natural SCSs as fillers, with wood chips being one of the most commonly used materials. Given the heterogeneity in application locations and substantial variations in water quality and volume, relevant studies have focused on the influence of temperature and HRT on denitrification and the measures required to overcome denitrification inhibition. Other key research directions include the age of filling materials (Ghane et al., 2018), decomposition and degradation of fillers (Seres et al., 2018), and leaching characteristics of DOC (Abusallout and Hua, 2017). 3.3 Surface water The input of pollutants beyond the carrying capacity has led to the aggravation of surface water eutrophication. For large water bodies with low pollutant concentrations, SCS-based denitrification technologies are a promising method to remove excessive nitrate-nitrogen and bring less side effect to water body (Chang et al., 2016). By adopting pretreated corn cobs, rice straw, and rice hulls as SCSs, Feng et al. (Xie et al., 2017) compared the impact of different carbon sources and pretreatment methods on SCS-based denitrification and analyzed the differences in effluent nitrogen pollutant concentration and microbial communities. They found that SCSs pretreated with acid or alkali achieved higher denitrification rates and lower effluent concentrations of ammonia-nitrogen and nitrites. In another study, Feng et al. (Feng et al., 2019) developed solid-phase denitrification systems using alkalipretreated rice husks, pomelo peels, and durian peels as biodegradable carriers for the simultaneous nitrification and denitrification of ammonia-nitrogen-polluted wastewater. High nitrogen removal rates (0.56–0.68 mg NH4+-N·(L·h)-1) and the identification of multiple new aerobic denitrifiers were realized. Another typical application of SCS-based denitrification technologies is in the removal of nitrogen pollutants in constructed wetlands. Jia et al. (Jia et al., 2019) utilized agricultural wastes (wheat straw) as carbon sources for the removal of nitrogen pollutants in a constructed wetland. The average dissolved organic carbon release rate was 5.24 mg·(g·d)−1, and three months assessment revealed TN removal efficiencies of 66.75–93.67 %. The DOM generated from the various agricultural wastes mainly consisted of humic and fulvic acid-like compounds. In another study, Shen et al. prepared cornstarch/PCL blends for use as SCSs in constructed wetlands. They found that the average denitrification rate and nitrate removal efficiency were 0.069 kg·(m3·d)-1 and 98.23 % (25℃, 72 h HRT), respectively, and the major component of DOM was polysaccharides which mainly consisted of reducing sugar (Shen et al., 2015). Si et al. selected wheat straw, cotton, PBS, and newspaper as external carbon sources for the comparison of NO3--N and TN removal rates under low and high temperatures, and found that newspaper achieved the highest removal rates under all temperature conditions (Si et al., 2018). Subsequently, 16S rRNA metagenomic sequencing was employed to investigate the influence of different SCSs on the structure and function of bacterial communities. Liu et al. constructed a wetland using PBS as the SCS for the treatment of ammonianitrogen-polluted wastewater under aerated conditions (Liu et al., 2018c). They found that TN removal rates of up to 99 % could be achieved, and simultaneous nitrification and denitrification was the main microbial nitrogen removal pathway. In addition to investigating nitrate-nitrogen removal, studies with respect to surface water bodies, including constructed wetlands, have mainly focused on ammonia-nitrogen and TN removal efficiencies and the simultaneous nitrification and denitrification process in the presence of SCSs. Furthermore, the impact of different carbon sources, temperatures, and DO levels on removal efficiencies and the DOM characteristics and functional microorganisms in effluents have also been frequently studied. 3.4 Groundwater The pollution of groundwater by nitrate-nitrogen is extremely severe in China (Ma et al., 2012). With the application of nitrogen fertilizers and the haphazard discharge of domestic sewage, nitrogen pollutants migrate to groundwater through the infiltration of surface runoff. Consequently, the adoption of SCS-based denitrification technologies for groundwater purification has received considerable attention. Chu et al. used a PHBV and bamboo powder blend as a carbon source and biofilm carrier in a packed-bed reactor for nitrate removal in groundwater (pump to surface). They found that the reactors achieved a rapid start-up without external inocula, nitrate removal efficiencies of up to 87.4 %, and less adverse effects in terms of nitrite accumulation (0.5 mg/L) and DOC release (10.5 mg/L) (Chu and Wang, 2016). When Xie et al. utilized a PHA/cellulose blend as a slow-release carbon material for the removal of nitrates from groundwater, they found that the blend exhibited excellent nitrate removal efficiency and less adverse effects in terms of nitrite accumulation during stable operations (Xie et al., 2017). In another study, Ye et al. developed a PHBV and ceramsite based permeable reactive barrier system, which was used in packed-bed reactors for the treatment of groundwater polluted with nitrate-nitrogen (Ye et al., 2017). Results of a continuous experiment conducted over a 35 day period indicated that more than 95 % of nitrate-nitrogen was removed and a maximum denitrification rate of 241 mg N·(L·d)-1 was achieved. Furthermore, they found that shortening the HRT significantly reduced the release of DOC. Jin et al. constructed a sawdust/pyrite mixotrophic denitrification reactor and analyzed the influence of sawdust dosage and HRT on reactor performance for in situ groundwater remediation (Jin et al., 2019). They found that an overdosage of sawdust increased nitrite-nitrogen and ammonia-nitrogen accumulation, and increasing the HRT from 12 to 24 h did not significantly enhance removal efficiency. The main aspects of interest in studies related to SCS-based groundwater treatment are similar to those of ordinary wastewater treatment and include the influence of the nitrate-nitrogen load, HRT, temperature, and carbon source dose on denitrification efficiency. Effluent parameters of interest mainly include nitrogen pollutant concentration, DOC concentration, and microbial community structure. 3.5 Summary of SCSs applications Table 2 provides a summary of the applications of SCSs in different target water bodies. Table 2. Characteristics of studies on the application of SCSs in different target water bodies Table 2 illustrates that differences in the characteristics and treatment requirements of the various target water bodies result in different carbon sources, process characteristics, factors of interest, and effluent parameters. For ordinary wastewater treatment, which is primarily aimed towards pollutant removal, effluent quality indicators are the primary concern. Furthermore, operating temperature and HRT and the accumulation of byproducts (e.g., DOC, nitrite-nitrogen, and ammonia nitrogen) in the effluent should also be examined. Consequently, studies on the application of SCS-based denitrification to ordinary wastewater treatment have mainly focused on achieving optimal effluent quality through parameter control. For recirculating aquaculture systems, effluent quality requirements are more stringent and treatment costs are usually higher compared to ordinary wastewater treatment. Consequently, synthetic SCSs with a low likelihood of secondary pollution, rapid carbon release, and high denitrification efficiency are commonly used. The influence of temperature on denitrification efficiency was rarely investigated with respect to recirculating aquaculture systems because the temperature is typically maintained within a certain range for aquatic product survival; however, the aquatic product yield was a key indicator. For agricultural subsurface drainage systems, natural agricultural and forestry wastes are the most common primary carbon sources due to the adaptability to local conditions and cost requirements. Research in this area is relatively well-established, with a key issue being the selection of the appropriate HRT to address large fluctuations in the quality and volume of drainage water. Because these drainage systems are mainly located in the open, reducing the constraints imposed by low temperatures on denitrification efficiency is also a key concern. Other areas of interest include denitrification efficiency at low HRT, byproduct concentrations in the effluent, carbon source operating life, and the simultaneous removal of nitrogen and phosphorus. Surface water is characterized by low pollutant concentrations and high flow rates, limiting microorganism enrichment, the formation of an adequate supply of localized carbon sources, and the denitrification ability of the microorganisms. This problem can be effectively resolved through the adoption of SCS-based denitrification technologies. Most existing studies have focused on conventional influencing factors (e.g. type and quantity of the carbon source and nitrogen pollutant load) and effluent quality parameters. Given the geographical and environmental characteristics of surface water, many researchers have also explored the influence of DO on the performance of carbon sources in surface water denitrification. In groundwater, which is characterized by low concentrations of easily oxidizable organic carbon and low temperatures, denitrification rates under natural conditions are usually lower (Wu, 2002). The microbial growth induced by the dosing of nutrient solutions in groundwater leads to a reduction in the pores of the water-containing medium, which consequently results in blockages; hence, the adoption of SCS-based denitrification technologies (especially denitrification barriers) has provided a novel means of nutrient supply. Existing studies on groundwater have mainly focused on the influences of nitrate-nitrogen load, HRT, and carbon source dosage on denitrification efficiency. Furthermore, the functional genes and microbial community structures related to the denitrification process have also been explored in research on the various target water bodies. 4 Meta-analysis on synthetic SCS-based denitrification In order to better understand the effect of different SCSs and reaction conditions on the denitrification efficiency, we applied meta-analysis on the published literature in the area of solidphase denitrification in recent years. Since the denitrification rates of synthetic carbon sources are much larger than that of raw carbon sources, in this study, we focus on the application of synthetic carbon source on nitrate removal. The data came from published journal articles that presented nitrate removal rates from flowthrough, synthesized SCS-based denitrification bed or lab-scale column reactors with nitrate as the main target containment. We searched for literature in the database of Web of Science with synthesized carbon sources (i.e. PBS, PCL, PHA, PHB, PHBV and PLA) and denitrification. Literature without long-term stable operation and key experimental data were excluded. 23 papers published focusing on synthesized carbon source based solid phase denitrification in recent three years were finally analyzed in our study. The core measure of solid-phase denitrification in our study was denitrification rates (DR) in the units of nitrate removal per volume of bioreactor per time (gNL-1d-1), and necessary calculation were applied using other information from papers when DR was not given directly. The data required for meta-analysis in our study were the mean value of DR and the corresponding standard deviation (SD). Based on our former discussion and available data, we chose carbon source type (CS type), carbon source species (CS species), influent N concentration, HRT and water temperature as target factors, which were further categorized into two or three levels, adapted from the meta analysis study on woodchip denitrification reactors (Addy et al., 2016). CS type is categorized into ‘mix CS’ (mixed synthetic carbon source or the mixture of synthetic and raw carbon sources) and ‘single CS’; three kinds of synthetic carbon source (i.e. PHBV, PHBV/PLA, PCL) with abundant experimental data are analyzed; Influent N concentration is divided into low, intermediate and high categories split by 20 and 50 mgN/L; HRT and water temperature are categorized in similar manner, split by 2 h and 5 h, 22℃ and 25℃, respectively. The actual data on mean removal rate, SD, number of studies and the classification of each factors are listed in Table S1. The response ratio (lnR) and the response variance (VlnR) were calculated (Addy et al., 2016) and MetaWin 2.0 was used for the calculation of nitrate removal rate effect size and its standard deviation. Forest plots of the meta-analysis results were shown in Figure 2. Figure 2. Mean nitrate removal effect size and 95% bias-corrected confidence interval by different categories of (a) CS type, (b) CS species, (c) Influent N concentration, (d) HRT and (e) Temperature. Numbers labeled in the figures are the mean value of effect size, and n represent for the number of studies in meta-analysis. There was no significant difference in nitrate removal rates between mix carbon source and single carbon source (Fig 2a), and while species of CS (PHBV, PHBV/PLA and PCL) were decisive as shown in Fig 2b. The mixing of carbon sources is generally for two purposes, one is to minimize the cost while ensuring the denitrification efficiency, and the other is to treat multiple pollutants simultaneously or in stages (Jiang et al., 2020, Yang et al., 2020a, Yang et al., 2020c). The small difference in denitrification performance showed that mixing of SCSs under specific water bodies and specific environments is worth further exploration. Nitrate removal rates were significantly effected by influent N concentration, as shown in Fig 2c. Reactors with high influent N concentration (>50 mgN/L) obtained higher denitrification rates than those with intermediate (20-50 mgN/L) and low (<20 mgN/L) influent N concentration. Higher nitrate concentration always resulted in larger reaction rates, and higher nitrate removal rates could also be obtained unless the exceeding of maximum denitrification capability of the system (Jiang et al., 2020, Xu et al., 2018a). HRT with different levels also significantly influenced nitrate removal rates (Fig 2d). However, the results showed that higher HRT (>5h) achieved the inferior performance on nitrate removal, which is contradictory to the results in a certain literature with the consideration of HRT levels (Ding et al., 2020, Yi et al., 2020, Zhang et al., 2021). This is mainly because, in some studies, researchers set a relatively long HRT in order to achieve a stable low nitrate effluent concentration (Feng et al., 2020a, Han et al., 2018, Lan et al., 2020). In fact, synthetic carbon sources often have short lag time and brilliant carbon release efficiency, and in many cases, HRT of 2h is sufficient for the thorough removal of nitrate (Fang et al., 2020, Shen et al., 2020). The nitrate removal rate effect sizes under different temperature were shown in Fig 2e. Low temperature (<20℃) significantly affect nitrate removal, where less COD release, nitrite accumulation and shift of denitrifying genus were detected at low-temperature (Shen et al., 2020, Xu et al., 2019b). Nevertheless, the high and intermediate categories are not significantly different in nitrate removal. There are two main reasons for this. First, the research literature analyzed mainly conducted lab-scale column reactors around room temperature (around 25℃), which was reasonable for the treatment of RAS, municipal waste water and surface water; second, the effect of low temperature on enzyme activities and microbial community related to carbon hydrolysis and denitrification are more obvious when it is below 15℃ (Jiang et al., 2020, Shen et al., 2020). Therefore, realizing high-efficiency denitrification under low temperature conditions (generally groundwater) is still a topic worthy of continued research. Synthetic solid-phase carbon sources have good application prospects for solid phase nitrification. The optimization of operation parameters (especially HRT), the mixing of synthetic carbon sources for actual complex water bodies and the further design of low temperature-tolerated reactors are important considerations for future study. 5 Advantage and disadvantage summary The eutrophication of water bodies remains a serious problem in many parts of the world; hence, research on the removal of nitrate-nitrogen pollution is extensive. In particular, research has focused on SCSs due to their ease of transport, low tendency for secondary pollution, long service life, and ease of management. In existing research, target water bodies for SCS-based denitrification include wastewater (including aquaculture wastewater), agricultural subsurface drainage, surface water, and groundwater. Relevant research on practical applications in agricultural subsurface drainage systems is extensive and well-established, while studies on practical applications in other water bodies are relatively scarce. Although SCS-based denitrification technologies have received widespread attention, they also possess certain shortcomings. Natural SCSs are inexpensive, easily acquirable, and have a long operating life; however, their applications are often limited due to unstable carbon release rates, excessive DOC release, and increased color intensity during the early stages of denitrification. Synthetic SCSs are readily utilized by microorganisms due to their strong bioaffinities, have a low tendency to cause secondary pollution due to their simple composition, and exhibit rapid carbon release rates and high denitrification efficiencies. Synthetic SCSs are superior to natural SCSs in most applications; however, they are expensive, have limited carbon release rates, and their denitrification performance is strongly influenced by temperature. While these factors currently limit the broader application of synthetic SCSs, it is anticipated that ongoing research will successfully address these issues. In conclusion, despite the presence of certain limitations, SCSbased denitrification technologies show promise for applications in many fields due to their superior advantages. 6 Conclusion Solid-phase carbon sources have good application prospects for solid phase nitrification. Research with respect to wastewater treatment has mainly been focused on the removal efficiency of nitrogen pollutants and DOC accumulation in the effluent, while studies on recirculating aquaculture systems have focused on product yield and water quality parameters. Agricultural subsurface drainage system research was extensive, and focused on natural SCSs and the influence of temperature and HRT on denitrification efficiency. The primary aspect of surface water research was the influence of DO on denitrification efficiency, while studies on groundwater were mainly focused on the influence of nitrate-nitrogen load, HRT, and carbon source dosage on denitrification efficiency. The optimization of operation parameters (especially HRT), the hybrid application of synthetic carbon sources and the further design of low temperature-tolerated reactors are worthy of continued study. Acknowledgement This work was supported by the National Natural Science Foundation of China (Grant No. 51809195), Postdoctoral Science Foundation of China (No. 2018M642083) and National Water Pollution Control and Treatment Science and Technology Major Project of China (Nos. 2017ZX07204004 and 2017ZX07204002). References Abusallout, I. and Hua, G., 2017. Characterization of dissolved organic carbon leached from a woodchip bioreactor. Chemosphere. 183, 36-43. Addy, K., et al., 2016. Denitrifying Bioreactors for Nitrate Removal: A Meta-Analysis. Journal of Environmental Quality. 45(3), 873-881. Bao, W., et al., 2019. Generation, characterization, perniciousness, removal and reutilization of solids in aquaculture water: a review from the whole process perspective. Reviews in Aquaculture. 11(4), 1342-1366. Boley, A. and Müller, W.-R., 2005. Denitrification with polycaprolactone as solid substrate in a laboratory-scale recirculated aquaculture system. Water Science and Technology. 52(10-11), 495502. Boley, A., et al., 2000. Biodegradable polymers as solid substrate and biofilm carrier for denitrification in recirculated aquaculture systems. Aquac. Eng. 22(1-2), 75-85. Canfield, D. E., et al., 2010. The Evolution and Future of Earth's Nitrogen Cycle. Science. 330(6001), 192-196. Chang, J., et al., 2016. Remediation of nitrate-contaminated wastewater using denitrification biofilters with straws of ornamental flowers added as carbon source. Water Science and Technology. 74(2), 416-423. Chen, X., et al., 2013. Regional Control of Groundwater Nitrogen Contamination. Geological Science and Techology Information. 32(6), 130. Cheng, H.-Y., et al., 2020. Aerobic denitrification performance and nitrate removal pathway analysis of a novel fungus Fusarium solani RADF-77. Bioresour. Technol. 295, 122250. Christianson, L. E., et al., 2012. A practice-oriented review of woodchip bioreactors for subsurface agricultural drainage. Appl. Eng. Agric. 28(6), 861-874. Christianson, L. E., et al., 2016. Denitrifying bioreactor clogging potential during wastewater treatment. Water Res. 105, 147-156. Chu, L. and Wang, J., 2011a. Comparison of polyurethane foam and biodegradable polymer as carriers in moving bed biofilm reactor for treating wastewater with a low C/N ratio. Chemosphere. 83(1), 63-68. Chu, L. and Wang, J., 2011b. Nitrogen removal using biodegradable polymers as carbon source and biofilm carriers in a moving bed biofilm reactor. Chemical Engineering Journal. 170(1), 220225. Chu, L. and Wang, J., 2013. Denitrification performance and biofilm characteristics using biodegradable polymers PCL as carriers and carbon source. Chemosphere. 91(9), 1310-1316. Chu, L. and Wang, J., 2016. Denitrification of groundwater using PHBV blends in packed bed reactors and the microbial diversity. Chemosphere. 155, 463-470. Chun, J. A., et al., 2010. Estimation of flow and transport parameters for woodchip-based bioreactors: II. field-scale bioreactor. Biosystems Engineering. 105(1), 95-102. Chun, J. A., et al., 2009. Estimation of flow and transport parameters for woodchip-based bioreactors: I. laboratory-scale bioreactor. Biosystems Engineering. 104(3), 384-395. David, M. B., et al., 2016. Temperature and Substrate Control Woodchip Bioreactor Performance in Reducing Tile Nitrate Loads in East-Central Illinois. J. Environ. Qual. 45(3), 822829. Deng, Y.-L., et al., 2017. The impact of DO and salinity on microbial community in poly(butylene succinate) denitrification reactors for recirculating aquaculture system wastewater treatment. AMB Express. 7(1), 113. Ding, W., et al., 2020. Effective control of the carbon release of starch/polyvinyl alcohol based on a polyamide coating in solid-phase denitrification. Environmental Science-Water Research & Technology. 6(12), 3293-3305. dos Santos, A. J., et al., 2018. From Obtaining to Degradation of PHB: A Literature Review. Part II. Ingeniería y Ciencia. 14(27), 207-228. Duan, L. a., et al.,2016. Denitrification performance using biodegradable polymer as carbon source to treat nitrified swine wastwater. 2016 ASABE Annual International Meeting. St. Joseph, MI, ASABE: 1. Fan, Z.-x. and Wang, J.-l., 2009. Denitrification using polylactic acid as solid carbon source. Huan jing ke xue= Huanjing kexue. 30(8), 2315-2319. Fan, Z., et al., 2012. Biological nitrate removal using wheat straw and PLA as substrate. Environmental Technology. 33(21), 2369-2374. Fang, D., et al., 2020. Polymer substrate reshapes the microbial assemblage and metabolic patterns within a biofilm denitrification system. Chemical Engineering Journal. 387. Feng, L., et al., 2019. Nitrification and aerobic denitrification in solid phase denitrification systems with various biodegradable carriers for ammonium-contaminated water purification. Journal of Chemical Technology & Biotechnology. 94(11), 3569-3577. Feng, L., et al., 2020a. Response of denitrifying community, denitrification genes and antibiotic resistance genes to oxytetracycline stress in polycaprolactone supported solid-phase denitrification reactor. Bioresource Technology. 308. Feng, L., et al., 2020b. Response of denitrifying community, denitrification genes and antibiotic resistance genes to oxytetracycline stress in polycaprolactone supported solid-phase denitrification reactor. Bioresour. Technol. 308, 123274. Feng, Y., et al., 2013. New types of extra carbon sources for denitrification. Modern Chemical Industry. 33(10), 52-57. Forrest, A. K., et al., 2010. Effects of temperature and pretreatment conditions on mixed-acid fermentation of water hyacinths using a mixed culture of thermophilic microorganisms. Bioresource Technology. 101(19), 7510-7515. Ghane, E., et al., 2018. Carbon Quality of Four-Year-Old Woodchips in a Denitrification Bed Treating Agricultural Drainage Water. Trans. ASABE. 61(3), 995-1000. Gomez, M. A., et al., 2000. Influence of carbon source on nitrate removal of contaminated groundwater in a denitrifying submerged filter. J. Hazard. Mater. 80(1-3), 69-80. Guo, Y. D., et al., 2017. Effects of hydraulic retention time (HRT) on denitrification using waste activated sludge thermal hydrolysis liquid and acidogenic liquid as carbon sources. Bioresour. Technol. 224, 147-156. Gutierrez-Wing, M. T., et al., 2012. Evaluation of polyhydroxybutyrate as a carbon source for recirculating aquaculture water denitrification. Aquacultural Engineering. 51, 36-43. Haihong, Z., et al., 2006. Denitrification Using PBS as Carbon Source and Biofiim Supporter: Effect of pH. Chinese journal of environmental science. 27(2), 290-293. Han, F., et al., 2018. Performance, microbial community and fluorescent characteristic of microbial products in a solid-phase denitrification biofilm reactor for WWTP effluent treatment. Journal of Environmental Management. 227, 375-385. Hang, Q. Y., et al., 2016. Application of plant carbon source for denitrification by constructed wetland and bioreactor: review of recent development. Environmental Science and Pollution Research. 23(9), 8260-8274. He, S., et al., 2018. Effect of hydraulic retention time on nitrogen removal and functional gene quantity/transcription in biochar packed reactors at 5 degrees C: A control-strategy study. Bioresource Technology. 264, 400-405. Healy, M. G., et al., 2012. Nitrate removal rate, efficiency and pollution swapping potential of different organic carbon media in laboratory denitrification bioreactors. Ecological Engineering. 40, 198-209. Hocking, P. J., et al., 1996. Enzymatic degradation of single crystals of bacterial and synthetic poly(beta-hydroxybutyrate). Macromolecules. 29(7), 2472-2478. Honda, Y. and Osawa, Z., 2002. Microbial denitrification of wastewater using biodegradable polycaprolactone. Polymer Degradation and Stability. 76(2), 321-327. Hu, R., et al., 2019. Effects of carbon availability in a woody carbon source on its nitrate removal behavior in solid-phase denitrification. Journal of Environmental Management. 246, 832839. Jafari, S. J., et al., 2015. High-rate biological denitrification in the cyclic rotating-bed biological reactor: Effect of COD/NO3-, nitrate concentration and salinity and the phylogenetic analysis of denitrifiers. Bioresour. Technol. 197, 482-488. Ji, F., et al., 2017. Denitrification performance of solid-phase denitrification biofilter and biochemical characteristics along its height. Chinese Journal of Environmental Engineering. 11(3), 1347-1354. Jia, L., et al., 2019. Exploring Utilization of Recycled Agricultural Biomass in Constructed Wetlands: Characterization of the Driving Force for High-Rate Nitrogen Removal. Environ. Sci. Technol. 53(3), 1258-1268. Jiang, L., et al., 2020. Denitrification performance and microbial diversity using starchpolycaprolactone blends as external solid carbon source and biofilm carriers for advanced treatment. Chemosphere. 255. Jin, S., et al., 2019. Effect of sawdust dosage and hydraulic retention time (HRT) on nitrate removal in sawdust/pyrite mixotrophic denitrification (SPMD) systems. Environmental Science: Water Research & Technology. 5(2), 346-357. Kessler, F., et al., 2014. Biodegradation improvement of poly(3-hydroxy-butyrate) films by entomopathogenic fungi and UV-assisted surface functionalization. Journal of Photochemistry and Photobiology B-Biology. 130, 57-67. Kim, J. S., et al., 2016. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresource Technology. 199, 42-48. Krause Camilo, B., 2016. Bioreactor reduces atrazine and nitrate in tile drain waters. Ecol. Eng. 86, 269-278. Lan, Z., et al., 2020. Comparative analysis of denitrification performance, denitrifying community and functional genes to oxytetracycline exposure between single and hybrid biodegradable polymers supported solid-phase denitrification systems. Biodegradation. 31(4-6), 289-301. Li, H., et al., 2019. Porous solid carbon source-supported denitrification in simulated mariculture wastewater. Environmental Technology, 1-8. Li, J., et al., 2012. Denitrification Performance of a Packed Bed Reactor Using Solid Carbon Source. Journal of Agro-Environment Science. 31(6), 1230-1235. Li, P., et al., 2016. Tertiary nitrogen removal for municipal wastewater using a solid-phase denitrifying biofilter with polycaprolactone as the carbon source and filtration medium. Water Research. 93, 74-83. Li, R., et al., 2017. Nitrate removal efficiency of a mixotrophic denitrification wall for nitratepolluted groundwater in situ remediation. Ecol. Eng. 106, 523-531. Liang, J., et al., 2015. Investigation of biological denitrification using biodegradable polymers cascade mini ring as carbon source. Chinese Journal of Environmental Engineering. 9(2), 633-638. Liu, D., et al., 2018a. Poly(butylene succinate)/bamboo powder blends as solid-phase carbon source and biofilm carrier for denitrifying biofilters treating wastewater from recirculating aquaculture system. Scientific Reports. 8(1), 3289. Liu, D., et al., 2018b. Poly(butylene succinate)/bamboo powder blends as solid-phase carbon source and biofilm carrier for denitrifying biofilters treating wastewater from recirculating aquaculture system. Scientific Reports. 8. Liu, H., et al., 2018c. Microbial nitrogen removal of ammonia wastewater in poly (butylenes succinate)-based constructed wetland: effect of dissolved oxygen. Applied Microbiology and Biotechnology. 102(21), 9389-9398. Liu, Y., et al., 2019. Nitrogen removal in a combined aerobic granular sludge and solid-phase biological denitrification system: System evaluation and community structure. Bioresour. Technol. 288, 121504. Lopardo, C. R. and Urakawa, H., 2019. Performance and microbial diversity of bioreactors using polycaprolactone and polyhydroxyalkanoate as carbon source and biofilm carrier in a closed recirculating aquaculture system. Aquaculture International. 27(5), 1251-1268. Lu, T., et al., 2017. Denitrification Performance of a Denitrifier-Augmented Packed-Bed Bioreactor with Solid Carbon Source. Acta Scientiarum Naturalium Universitatis Pekinensis. 53(5), 957-963. Lucas, N., et al., 2008. Polymer biodegradation: Mechanisms and estimation techniques – A review. Chemosphere. 73(4), 429-442. Luo, G., et al., 2019. Comparison of nitrate-removal efficiency and bacterial properties using PCL and PHBV polymers as a carbon source to treat aquaculture water. Aquaculture and Fisheries. Luo, G., et al., 2016. Effect of dissolved oxygen on nitrate removal using polycaprolactone as an organic carbon source and biofilm carrier in fixed-film denitrifying reactors. Journal of Environmental Sciences. 43, 147-152. Ma, H., et al., 2012. Status of Nitrate Nitrogen Contamination of Groundwater in China. Journal of Soil Science. 43(6), 1532-1536. Mateju, V., et al., 1992. BIOLOGICAL WATER DENITRIFICATION - A REVIEW. Enzyme Microb. Technol. 14(3), 170-183. Muller, W. R., et al., 1992. Aspects of PHA (poly-B-hydroxy-butyric-acid) as an h-donator for denitrification in water treatment processes. Water Supply. 10, 79–90. Ovez, B., et al., 2006. Biological denitrification in drinking water using Glycyrrhiza glabra and Arunda donax as the carbon source. Process Biochemistry. 41(7), 1539-1544. Podduturi, R., et al., 2020. Geosmin fluctuations and potential hotspots for elevated levels in recirculated aquaculture system (RAS): A case study from pikeperch (Stizostedion lucioperca) production in Denmark. Aquaculture. 514, 734501. Qiu, T., et al., 2017. Bacterial community dynamics in a biodenitrification reactor packed with polylactic acid/poly (3-hydroxybutyrate-co-3-hydroxyvalerate) blend as the carbon source and biofilm carrier. Journal of Bioscience and Bioengineering. 123(5), 606-612. Rout, P. R., et al., 2017. Assessing Possible Applications of Waste Organic Solid Substances as Carbon Sources and Biofilm Substrates for Elimination of Nitrate Toxicity from Wastewater. Journal of Hazardous, Toxic, and Radioactive Waste. 21(3), 04016027. Ruan, Y.-J., et al., 2016. Simultaneous ammonia and nitrate removal in an airlift reactor using poly(butylene succinate) as carbon source and biofilm carrier. Bioresource Technology. 216, 10041013. Schipper, L. A., et al., 2010. Denitrifying bioreactors—An approach for reducing nitrate loads to receiving waters. Ecological Engineering. 36(11), 1532-1543. Seres, M., et al., 2018. The impact of woodchip-gravel mixture on the efficiency and toxicity of denitrification bioreactors. The Science of the total environment. 647, 888-894. Shah, A. A., et al., 2008. Biological degradation of plastics: A comprehensive review. Biotechnology Advances. 26(3), 246-265. Shen, Q., et al., 2020. The influence mechanism of temperature on solid phase denitrification based on denitrification performance, carbon balance, and microbial analysis. Science of the Total Environment. 732. Shen, Z., et al., 2013. Denitrification performance and microbial diversity in a packed-bed bioreactor using biodegradable polymer as carbon source and biofilm support. Journal of Hazardous Materials. 250-251, 431-438. Shen, Z., et al., 2015. Enhanced removal of nitrate using starch/PCL blends as solid carbon source in a constructed wetland. Bioresource Technology. 175, 239-244. Si, Z., et al., 2018. Intensified heterotrophic denitrification in constructed wetlands using four solid carbon sources: Denitrification efficiency and bacterial community structure. Bioresource Technology. 267, 416-425. Steidl, J., et al., 2019. Nitrogen retention efficiency of a surface-flow constructed wetland receiving tile drainage water: A case study from north-eastern Germany. Agriculture, Ecosystems & Environment. 283, 106577. Sun, G., et al., 2019a. Enhanced removal of nitrate and refractory organic pollutants from biotreated coking wastewater using corncobs as carbon sources and biofilm carriers. Chemosphere. 237, 124520. Sun, H., et al., 2019b. Simultaneous removal of nitrogen and pharmaceutical and personal care products from the effluent of waste water treatment plants using aerated solid-phase denitrification system. Bioresource Technology. 287, 121389. Sun, H., et al., 2020. Enhanced simultaneous nitrification and denitrification performance in a fixed-bed system packed with PHBV/PLA blends. International Biodeterioration & Biodegradation. 146, 104810. Takahashi, M., et al., 2011. Nitrate Removal Efficiency and Bacterial Community Dynamics in Denitrification Processes Using Poly (L-lactic acid) as the Solid Substrate. Microbes Environ. 26(3), 212-219. Wang, J. and Chu, L., 2016. Biological nitrate removal from water and wastewater by solidphase denitrification process. Biotechnology Advances. 34(6), 1103-1112. Wu, B., et al., 2021. Mechanism insights into polyhydroxyalkanoate-regulated denitrification from the perspective of pericytoplasmic nitrate reductase expression. Science of the Total Environment. 754. Wu, W., et al., 2012. Biological denitrification with a novel biodegradable polymer as carbon source and biofilm carrier. Bioresource Technology. 118, 136-140. Wu, W., et al., 2013. Denitrification performance and microbial diversity in a packed-bed bioreactor using PCL as carbon source and biofilm carrier. Applied Microbiology and Biotechnology. 97(6), 2725-2733. Wu, Y., 2002. Denitrification in groundwater systems. Techniques and equipment for environmental pollution control. 3(3), 27-31. Xie, Y., et al., 2017. Slowly released carbon source from composite materials system for removing nitrate pollution in groundwater. Rsc Advances. 7(17), 10215-10220. Xiong, R., et al., 2019. Biological denitrification using polycaprolactone-peanut shell as slowrelease carbon source treating drainage of municipal WWTP. Chemosphere. 235, 434-439. Xiong, R., et al., 2020. Comparison of agricultural wastes and synthetic macromolecules as solid carbon source in treating low carbon nitrogen wastewater. Science of the Total Environment. 739. Xu, Z., et al., 2018a. Effect of different carbon sources on denitrification performance, microbial community structure and denitrification genes. Science of the Total Environment. 634, 195-204. Xu, Z., et al., 2019a. Biological denitrification using PHBV polymer as solid carbon source and biofilm carrier. Biochemical Engineering Journal. 146, 186-193. Xu, Z., et al., 2019b. Effect of temperature on tertiary nitrogen removal from municipal wastewater in a PHBV/PLA-supported denitrification system. Environmental Science and Pollution Research. 26(26), 26893-26899. Xu, Z., et al., 2018b. PHBV polymer supported denitrification system efficiently treated high nitrate concentration wastewater: Denitrification performance, microbial community structure evolution and key denitrifying bacteria. Chemosphere. 197, 96-104. Xu, Z. X., et al., 2009. Biological Denitrification Using Corncobs as a Carbon Source and Biofilm Carrier. Water Environ. Res. 81(3), 242-247. Yang, F. and Wu, W., 2014. Biological denitrification using PHBV as carbon source and biofilm carrier. China Environmental Science. 34(7), 1703-1708. Yang, Z., et al., 2020a. Intensified simultaneous nitrification and denitrification performance in integrated packed bed bioreactors using PHBV with different dosing methods. Environmental Science and Pollution Research. 27(17), 21560-21569. Yang, Z., et al., 2020b. Nitrogen removal performance in pilot-scale solid-phase denitrification systems using novel biodegradable blends for treatment of waste water treatment plants effluent. Bioresource Technology, 122994. Yang, Z., et al., 2020c. Nitrogen removal performance in pilot-scale solid-phase denitrification systems using novel biodegradable blends for treatment of waste water treatment plants effluent. Bioresource Technology. 305. Yao, Z., et al., 2019. Development of a hybrid biofilm reactor for nitrate removal from surface water with macrophyte residues as carbon substrate. Ecol. Eng. 128, 1-8. Ye, L. T., et al., 2017. Denitrification of nitrate-contaminated groundwater in columns packed with PHBV and ceramsites for application as a permeable reactive barrier. Water Science and Technology-Water Supply. 17(5), 1241-1248. Yi, C., et al., 2020. Renovated filter filled with poly-3-hydroxybutyrateco-hydroxyvalerate and granular activated carbon for simultaneous removal of nitrate and PPCPs from the secondary effluent. Science of the Total Environment. 749. Zhang, H. W., et al., 2016a. Biological nitrate removal using a food waste-derived carbon source in synthetic wastewater and real sewage. J. Environ. Manage. 166, 407-413. Zhang, N., et al., 2016b. Growth, digestive enzyme activity and welfare of tilapia (Oreochromis niloticus) reared in a biofloc-based system with poly-beta-hydroxybutyric as a carbon source. Aquaculture. 464, 710-717. Zhang, Q., et al., 2016c. Effects of physicochemical properties of poly-epsilon-caprolactone on nitrate removal efficiency during solid-phase denitrification. Chemical Engineering Journal. 283, 604-613. Zhang, Q., et al., 2016d. Optimization of nitrate removal from wastewater with a low C/N ratio using solid-phase denitrification. Environmental Science and Pollution Research. 23(1), 698-708. Zhang, S., et al., 2021. Effect of filling ratio and backwash on performance of a continuousflow SPD reactor packed with PCL as carbon source. Water environment research : a research publication of the Water Environment Federation. Zhang, S., et al., 2018. Bioaugmentation with Diaphorobacter polyhydroxybutyrativorans to enhance nitrate removal in a poly (3-hydroxybutyrate-co-3-hydroxyvalerate)-supported denitrification reactor. Bioresource Technology. 263, 499-507. Zhang, S. S., et al., 2017. Heterotrophic nitrification and aerobic denitrification by Diaphorobacter polyhydroxybutyrativorans SL-205 using poly(3-hydroxybutyrate-co- 3hydroxyvalerate) as the sole carbon source. Bioresour. Technol. 241, 500-507. Zhang, Y., et al., 2014. Tracing nitrate pollution sources and transformation in surface- and ground-waters using environmental isotopes. Science of the Total Environment. 490, 213-222. Zhang, Z., et al., 2020. Recent advances in partial denitrification in biological nitrogen removal: From enrichment to application. Bioresource Technology. 298, 122444. Zhao, J., et al., 2020. Denitrification behavior in a woodchip-packed bioreactor with gradient filling for nitrate-contaminated water treatment. Biochemical Engineering Journal. 154, 107454. Zhenxing, F. and Jianlong, W., 2008. Denitrification at low temperatures using BDPs as the solid carbon source in a packed bed reactor. Journal of Tsinghua University. Science and Technology. 48(3), 436-439. Zhong, H., et al., 2020. Solid-phase denitrification for water remediation: processes, limitations, and new aspects. Critical Reviews in Biotechnology. 40(8), 1113-1130. Zhu, S.-M., et al., 2015. Biological denitrification using poly(butylene succinate) as carbon source and biofilm carrier for recirculating aquaculture system effluent treatment. Bioresource Technology. 192, 603-610. *Graphical Abstract *Highlights (for review : 3 to 5 bullet points (maximum 85 characters including spaces per bullet point) Highlights: - Nitrogen pollution in water bodies is a serious environmental concern Solid carbon sources are an effective denitrification treatment method Synthetic carbon sources were more effective compared to natural carbon sources Different water bodies required varied approaches to optimize denitrification Research progress in solid carbon source–based denitrification technologies for different target water bodies Feifan Zhang a, Chengjin Ma a, Xiangfeng Huang a, Jia Liu a, Lijun Lu a, Kaiming Peng a, Shiyang Li a a College of Environmental Science and Engineering, State Key Laboratory of Pollution Control and Resource Reuse, Ministry of Education Key Laboratory of Yangtze River Water Environment, Tongji University, Shanghai 200092, People’s Republic of China Correspondence information: Shiyang Li, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, People’s Republic of China. Tel: +86 021 65982399. Email: lishiyang@tongji.edu.cn Abstract: Nitrogen pollution in water bodies is a serious environmental issue which is commonly treated by various methods such as heterotrophic denitrification. In particular, solid carbon source (SCS)–based denitrification has attracted widespread research interest due to its gradual carbon release, ease of management, and long-term operation. This paper reviews the types and properties of SCSs for different target water bodies. While both natural (wheat straw, wood chips, and fruit shells) and synthetic (polybutylene succinate, polycaprolactone, polylactic acid, and polyhydroxyalkanoates) SCSs are commonly used, it is observed that the denitrification performance of the synthetic sources is generally superior. SCSs has been used in the treatment of wastewater (including aquaculture wastewater), agricultural subsurface drainage, surface water, and groundwater; however, the key research aspects related to SCSs differ markedly based on the target waterbody. These key research aspects include nitrogen pollutant removal rate and byproduct accumulation (ordinary wastewater); water quality parameters and aquatic product yield (recirculating aquaculture systems); temperature and hydraulic retention time (agricultural subsurface drainage); the influence of dissolved oxygen (surface waters); and nitrate-nitrogen load, HRT, and carbon source dosage on denitrification rate (groundwater). It is concluded that SCSbased denitrification is a promising technique for the effective elimination of nitrate-nitrogen pollution in water bodies. Keywords: solid carbon source, heterotrophic denitrification, nitrogen pollution, nitrogen removal, water treatment 1 Introduction A fast increase in human population and rapid developments in the industrial and agricultural sectors have resulted in nitrogen pollution–induced eutrophication of water bodies (Jafari et al., 2015, Liu et al., 2019). In natural water bodies, nitrogen existing in forms other than nitrate-nitrogen is gradually converted to nitrate-nitrogen by microorganisms, resulting in an increasingly severe accumulation of nitrate-nitrogen in water bodies (Canfield et al., 2010, Zhang et al., 2014). Water bodies containing excessive nitrate-nitrogen may cause damage to crop root systems and impact crop yields when used as water sources for agricultural irrigation (Steidl et al., 2019). Furthermore, drinking from polluted water bodies may cause toxic effects or even death in wildlife, and excessive nitrate-nitrogen intake in humans can result in methemoglobinemia (blue baby syndrome) or may cause toxic effects or even death in wildlife in extreme cases (Li et al., 2017). Studies have revealed that nitrogen pollution in groundwater exists in approximately 110 countries and has caused safety issues concerning drinking water globally (Chen et al., 2013, Feng et al., 2020b). In response to existing nitrogen pollution issues, many physicochemical and bioecological methods have been utilized for the removal of excess nitrate-nitrogen in water. Owing to the current technological progress, the application of carbon sources under anoxic conditions to achieve biological nitrogen removal through denitrification (Cheng et al., 2020, Zhang et al., 2017) has emerged as the mainstream method employed in water treatment. Treatment processes in traditional wastewater treatment plants usually involve the addition of external carbon sources to promote microbial denitrification. Commonly used external carbon sources mainly consist of water-soluble low-molecular-weight (LMW) organic compounds such as methanol, acetates, and LMW sugars (Feng et al., 2013). However, the addition of dissolved carbon sources to natural water bodies increases the organic pollution load and may even cause secondary pollution if the calculated dosage amounts are unreasonable. Furthermore, the need for dosing facilities is also a limiting factor in the widespread use of dissolved carbon sources. Conversely, solid carbon sources (SCSs) have attracted a considerable research interest due to their appropriate carbon release rates, favorable conditions for microbial biofilm growth, long carbon release duration, ease of management, and long-term operation(Yang et al., 2020b, Zhang et al., 2020). Currently, the most commonly used SCSs can be classified into two major categories, i.e, natural cellulosic materials and synthesized biodegradable polymers (BDPs). Target water bodies for SCS-based denitrification include wastewater (including aquaculture wastewater), agricultural subsurface drainage, surface water, and groundwater. To date, studies regarding the application of SCSs to different target water bodies under specific conditions and operating requirements have investigated the types and properties of commonly used carbon sources, process characteristics, influencing factors, and effluent parameters. In this paper, we provide an overview both lab and field studies of the types and operating principles of commonly used SCSs based on a review in this field. We identify and classify the denitrification principles of synthetic SCSs, commonly used SCSs, influencing factors, and effluent parameters. Meta-analysis was further applied for better understanding of the effect of different SCSs and reaction conditions on the denitrification efficiency. Finally, we summarize the limitations governing the use of SCSs to provide a scientific basis for the future development of SCS-based denitrification techniques. 2 Types, properties and utilization of SCSs 2.1 Commonly used SCSs Commonly used SCSs can be classified into three major categories: natural (cellulosic), synthetic (polymeric), and other SCSs (e.g., acidogenic liquids from food waste, hydrolyzed sludge and other reprocessed organic materials) (Guo et al., 2017, Zhang et al., 2016a). Due to the scarcity of relevant existing literature and applications, other SCSs have not been discussed in this paper. Most early applications of SCSs involved the use of natural carbon sources, including cellulosic agricultural and forestry wastes, such as corn cobs, corn stover, wheat straw, cardboard fibers, leaf litter, tree bark, wood chips, and fruit kernels, which were reported in previous studies (Chang et al., 2016, Christianson et al., 2012, Chun et al., 2009, Gomez et al., 2000). Natural SCSs have several advantages such as low cost and ease of acquisition. Because they consist primarily of agricultural and forestry wastes, they are commonly used as filling materials in denitrification located near the outflow of agricultural subsurface drainage systems. However, the wider application of natural SCSs in denitrification is limited by unstable carbon release rates, slow denitrification rates, excessive release of dissolved organic carbon (DOC), and increased color intensity in the effluent. Therefore, while the denitrification efficiencies of natural SCSs were reported more frequently in earlier studies, more recent literature has focused on the simultaneous removal of total nitrogen (TN) and nitrate-nitrogen (Si et al., 2018), aerobic denitrification (Cheng et al., 2020), characteristics of leached pollutants (Abusallout and Hua, 2017, Jia et al., 2019), and functional microbial communities (Hu et al., 2019). In recent years, the use of BDPs as synthetic SCSs has attracted considerable research attention. BDPs only decompose under the action of extracellular enzymes secreted by specific microorganisms; hence, they can avoid or mitigate many of the aforementioned problems associated with natural SCSs. BDPs refer to a class of high-molecular-weight (HMW) materials that are degraded or enzymatically hydrolyzed under biological action to generate LMW compounds which can be utilized by organisms. In addition to serving as carbon sources, BDPs as synthetic SCSs also act as carriers for the growth of denitrifying microorganisms (Zhang et al., 2016c; d) and have been used in the denitrification of water bodies since the 1990s. In 1992, Müller et al. reported the use of polyhydroxybutyrate (PHB) granules as an SCS for denitrification through the construction of a laboratory-scale up flow fixed-bed reactor (Muller et al., 1992). They found that the denitrification rate at 10 ºC was 11 mg·(L·h)-1, and cells co-immobilized with the PHB granules exhibited a higher denitrification rate compared to suspended cells. Currently, only a few types of synthetic SCSs are commonly applied to the denitrification of wastewater, including polylactic acid (PLA) (Fan et al., 2012), polycaprolactone (PCL) (Chu and Wang, 2011a; b, Li et al., 2016, Wu et al., 2013), polybutylene succinate (PBS) (Wang and Chu, 2016), and polyhydroxyalkanoates (PHAs) (Lopardo and Urakawa, 2019). Table 1 illustrates a summary of the denitrification efficiencies reported in a number of studies on SCSs. Table 1. Summary of denitrification performance of different solid carbon source (SCSs) reported in previous literature 2.2 Basic principles of synthetic SCS-based denitrification 2.2.1 Synthetic SCSs Utilization Anoxic biological denitrification usually takes place under the action of microorganisms and fungus. It involves biological redox reactions in which organic carbon sources and nitrates serve as electron donors and acceptors, respectively. This results in the reduction of nitrate to nitrogen, which is subsequently removed from the water body through the denitrification process. The overall process consists of the following steps: 𝑁𝑂3− → 𝑁𝑂2− → 𝑁𝑂 → 𝑁2 𝑂 → 𝑁2 However, only soluble biodegradable carbon source such as acetic acid, formic acid, and methanol can be directly utilized by denitrifying microorganisms during biological denitrification, whereas SCSs must be converted into LMW compounds prior to utilization by microorganisms. The utilization of BDPs as carbon sources and bacterial carriers for SCS based denitrification occurs under microbial action, with SCS biodegradation enabling the growth and metabolism of microorganisms attached to the surfaces of SCSs and the . Polymer degradation can be characterized as a process that results in the breakage of a large and complex molecule into smaller molecules (dos Santos et al., 2018). First, biofilms are formed through the attachment and growth of microorganisms on polymer surfaces; then, polymer chains are cleaved by extracellular enzymes, leading to the hydrolysis of the polymers into soluble LMW compounds (Hocking et al., 1996, Shah et al., 2008). Subsequently, LMW compounds enter functional microorganisms through semipermeable membranes and are utilized as carbon sources and electron donors. In short, the utilization of BDPs involves hydrolysis and denitrification, with the former being the rate- determining process (Takahashi et al., 2011). Figure 1. Schematic of the utilization of biodegradable polymers (BDPs) as carbon sources by denitrifying microorganisms Microbial degradation has various impacts on SCSs, including changes in chemical structure (Lucas et al., 2008), significant reductions in the polymer molecular weight, increased surface roughness, formation of perforations and pits, and mechanical deformation due to structural dissolution and breakdown. In turn, these impacts change the carbon release rate of the material. Microbial degradation also leads to a decrease in the crystalline phase content and a corresponding increase in readily hydrolysable amorphous content, resulting in hydrophilicity changes. 2.2.2 Basic mechanisms of synthetic SCS-based denitrification Müller et al. had reported the use of PHB (molecular formula: [C4H6O2]n) as a synthetic SCS (Muller et al., 1992). And PHB based denitrification reaction with nitrate ions as electron acceptors is as follows: 5[𝐶4𝐻6 𝑂2 ] + 18𝑁𝑂3 − → 9𝑁2 + 18𝐻𝐶𝑂3 − + 2𝐶𝑂2 + 6𝐻2𝑂 (1) According to Boley et al., by assuming a yield coefficient (Yx/s) of 0.45 g biomass/g PHB, the summarized denitrification equation including biomass formation when PHB is used as the carbon source can be expressed as (Boley et al., 2000): 0.494[𝐶4 𝐻6𝑂2 ] + 𝑁𝑂3 − → 0.415𝑁2 + 𝐻𝐶𝑂3 − + 0.130𝐶𝑂2 + 0.169[𝐶5 𝐻7𝑂2 𝑁] (2) + 0.390𝐻2 𝑂 where C5H7O2N is the molecular formula of the microbial cells. Because the molecular formulae of commonly used synthetic SCSs (PCL, PBS, PLA, and PHAs) can be represented as CxHyOz, the basic denitrification reaction for synthetic SCSs is as follows: 5[𝐶𝑥 𝐻𝑦 𝑂𝑧 ] + (4𝑥 + 𝑦 − 2𝑧)𝑁𝑂3 − → (2𝑥 + 𝑦 − − 𝑧)𝑁2 + (4𝑥 + 𝑦 − 2𝑧)𝐻𝐶𝑂3 + (𝑥 − 𝑦 + 2𝑧)𝐶𝑂2 2 (3) + (2𝑦 − 2𝑥 + 𝑧)𝐻2𝑂 Using Equation (3), the calculated mass of PHB required to reduce the unit mass of nitratenitrogen is 2.92 g PHB/g NO3--N when biomass formation is excluded and 3.03 g PHB/g NO3--N when biomass formation is considered. Based on theoretical calculations, the masses of glucose, methanol, and ethanol required to reduce the unit mass of nitrate-nitrogen when biomass formation is excluded are 2.68, 1.90, and 1.37 g/g NO3--N, respectively. When biomass formation is considered, the required masses of methanol and ethanol are 2.47 and 2.01 g/g NO3--N, respectively (Mateju et al., 1992). 2.3 Comparison of raw and synthetic SCSs Compared with liquid carbon sources, SCSs can be used as biofilm carriers for microorganisms, which are more convenient for transportation, operation and storage (Boley et al., 2000). In previous studies, most solid carbon sources can release organic matter needed in denitrification systems, especially in low C/N wastewater treatment. In terms of material sources, raw and synthetic SCSs come from multiple sources. Cellulosic agricultural and forestry wastes are the main components of raw SCSs. Synthetic SCSs are mainly produced by petrochemical engineering, while some studies focus on the utilization of endogenous accumulation of PHAs by microorganisms (He et al., 2018, Wu et al., 2021). With the development of material science, more and more new materials are used in sewage treatment. BDPs with excellent biocompatibility, biodegradability and non-toxicity, such as PCL and PBS, are ideal materials for environmental protection (Wu et al., 2012). The hydrolytic ability of carbon sources is regarded as a key factor of denitrification (Hang et al., 2016). Denitrification rate is inhibited when available carbon from carbon source is insufficient, while an over-release of carbon may on the contrary, cause carbon loss and organic pollution (Healy et al., 2012). Regarding the characteristics of carbon release, first-order kinetics can basically describe the carbon release process of all SCSs. Raw SCSs can release more organic matter, and synthetic SCSs have an advantage in the sustainability of carbon release. In order to take advantage of the advantages of both kinds of SCSs in terms of carbon release, some carbon sources mixed with raw and synthetic SCSs have been prepared (Jiang et al., 2020, Liu et al., 2018b, Xiong et al., 2019) . The hydrolysate of raw and synthetic SCSs are quite different. Raw SCSs often contain multiple components. Taking lignocellulosic materials as an example, they are mainly comprised of lignin, cellulose and hemicellulose. Among them, cellulose can be easily used by microorganisms and hydrolyzed into glucose, hemicellulose can also be used after enzymatic hydrolysis into small molecular organic matter, and lignin is difficult to be degraded (Forrest et al., 2010, Zhong et al., 2020). It has been reported that the denitrification efficiency of woody biomass is lower than that of herbaceous biomass due to its lignin content and natural structure in woody biomass (Kim et al., 2016). The hydrolysate of the synthetic SCSs is related to its own structure, and its degradation involves the joint effect of several processes. Taking PHB as an example, PHB can directly undergo abiotic hydrolysis in water because it contains -COO- group as a polyester. When PHB is used as SCS in a biological denitrification system, biodegradation of PHB also plays a significant role. Enzymes in the intracellular and extracellular matrix can disrupt long-chain polymer chains and hydrolyze oligomers, and these oligomers will be further hydrolyzed into polymer monomers after a short period of time (dos Santos et al., 2018, Kessler et al., 2014). Water temperature, pH value, microbial community and the supplement of nutrients will all affect the degradation process of SCSs. In terms of denitrification, both SCSs can promote denitrification in low C/N wastewater treatment. Due to the difference in carbon release patterns between the two types of carbon sources, and considering the sustainability of carbon release, synthetic SCSs are ideal slow-release carbon sources for low TN water treatment (such as groundwater and drinking water), raw SCSs may be a more ideal carbon source to enhance the denitrification of secondary wastewater (Chu and Wang, 2013, Xiong et al., 2020). 3 Application of SCSs in different target water bodies Currently, the target water bodies for SCS-based denitrification technologies include wastewater (Duan et al., 2016, Xu et al., 2018a, Yang et al., 2020b) (including aquaculture wastewater (Gutierrez-Wing et al., 2012, Zhang et al., 2016b)), agricultural subsurface drainage, (Christianson et al., 2016, Chun et al., 2010), surface water(Feng et al., 2019), and groundwater (Zhang et al., 2018). 3.1 Wastewater Studies on the application of SCSs to wastewater denitrification can be classified into two main categories; (1) advanced treatment of ordinary wastewater or wastewater treatment plant effluent and (2) purification of nitrogen pollutants in recirculating aquaculture systems. 3.1.1 Treatment of ordinary wastewater For wastewater with characteristically low C/N ratios, nitrogen removal is often achieved via dosing with external carbon sources. Generally, SCSs are used as filling materials and microbial carriers in fixed-bed reactors for the purification of wastewater by denitrification. In a study by Rout et al. (Rout et al., 2017), organic solid waste substances were used as carbon sources to investigate the influence of experimental parameters, such as influent nitrate concentration, hydraulic retention time (HRT), and bed depth, on denitrification efficiency. The found that a low HRT reduced nitrate removal efficiency, increased nitrite accumulation, and decreased effluent chemical oxygen demand (COD). When the influent nitrate concentration was 70, 50, and 30 mg/L, the effluent nitrate concentration could be maintained at < 10 mg/L for 31, 39, and 49 days, respectively, with the denitrification process following first-order reaction kinetics. Sun et al. (Sun et al., 2019a) utilized alkali-pretreated corn cobs as solid carbon sources and biofilm carriers for the removal of nitrates and refractory organic pollutants from coking wastewater. They found that the treatment process could concurrently achieve the stable removal of over 90 % of residual nitrate and the degradation of typical refractory organic matter. In another study, Duan et al. compared the performances of PBS, PHBV, and PCL as carbon sources for the treatment of nitrified swine wastewater (Duan et al., 2016). They found that the denitrification reaction time was shortest when PCL was used, with the nitrate removal rate exceeding 95 % after 20 days of cultivation, and total organic carbon (TOC) and NH4+-N were absent in the effluent. Xu et al. constructed a packed-bed bioreactor using a PHBV/PLA blend as a carbon source and biofilm carrier for the removal of ammonia-nitrogen, nitrite-nitrogen, and nitrate-nitrogen from the effluent of a secondary settling tank in an activated sludge wastewater plant (Xu et al., 2019b). They found that the nitrogen removal system effectively removed all three nitrogen pollutants. Furthermore, the nitrogen removal efficiency was influenced by temperature (the denitrification rate at 30 ºC was five times greater than at 10 ºC); however, higher temperatures also promoted TOC accumulation. Sun et al. (Sun et al., 2020) also used a PHBV/PLA blend for the purification of sewage treatment plant effluent, and achieved removal efficiency of 98.1 ± 2.9, 87.2 ± 6.8, and 89.3 ± 6.3 % for NH4+-N, NO3—N, and TN, respectively. These results indicate that the reactor system was capable of simultaneous nitrification and denitrification under appropriate aeration conditions. Previous studies on SCS-based wastewater treatment utilized both natural and synthetic SCSs and mainly focused on the impact of the type of carbon source, temperature, HRT, and pH on treatment efficiency. In light of the objectives of wastewater treatment, a significant number of relevant studies have also examined nitrite and ammonia-nitrogen accumulation and effluent TOC, whereas other studies have investigated changes in microbial communities and the abundance of functional genes. 3.1.2 Recirculating aquaculture systems Recirculating aquaculture systems have emerged as a novel aquaculture technology that involve the treatment of aquaculture wastewater and subsequent recycling and reuse of the treated water. The removal of nitrate-nitrogen represents a key step in the wastewater purification process (Bao et al., 2019, Podduturi et al., 2020). In a study by Luo et al. (Luo et al., 2019), the denitrification performance and bacterial properties of recirculating aquaculture systems using PCL and PHBV as SCSs were compared over a 102 day period. They found that the denitrification rates achieved with PCL and PHBV under influent nitrate-nitrogen concentrations of 81.1–132.75 mg/L and an influent flow rate of 1 L/h were 0.27 and 0.19 g·(L·d)-1, respectively. For the removal of the same mass of nitrate-nitrogen, the mass of PCL consumed was significantly lower than the mass of PHBV, and the effluent nitrate-nitrogen and ammonia-nitrogen concentrations achieved using PCL were also lower. Deng et al. investigated the influence of operating conditions such as dissolved oxygen (DO) concentration and salinity on nitrogen removal performance and microbial communities in a recirculating aquaculture system that utilized PBS as the carbon source (Deng et al., 2017). They found that salinity decreased the number and diversity of operational taxonomic units, while DO had no significant influence on the microbial community. Zhu et al. constructed a denitrification bioreactor using PBS as the carbon source and compared the denitrification performance using real and synthetic recirculating aquaculture system wastewater to determine the influence of salinity and nitrate concentration on heterotrophic denitrification (Zhu et al., 2015). They found that the nitrate volumetric removal rate increased with influent nitrate loading. Conversely, salinity had little influence on nitrate removal (an increase of salinity from 0‰ to 25‰ led to an increase of denitrification rate from 0.53 to 0.66 kg NO3--N·(m3·d)-1); however, it did increase the likelihood of excessive DOC and ammonia-nitrogen accumulation in the effluent. Li et al. prepared a novel beadshaped SCS using semen litchi (SL), poly (vinyl alcohol) (PVA), and sodium alginate (SA) as raw materials (Li et al., 2019). They found that the denitrification rate was up to 243.5 ± 7.08 mg N·(L·d)-1 when the beads were used in the SCS based denitrification of mariculture wastewater. Zhang et al. (Zhang et al., 2016b) introduced PHB as a denitrification carbon source into an aquaculture system in which 120 tilapias were reared. After 120 days of culture without water exchange, it was found that the nitrate-nitrogen concentration of the system was maintained at a certain level, thereby effectively avoiding the toxic effects of nitrate-nitrogen on aquatic animals. Existing studies have indicated that the SCSs used in most recirculating aquaculture systems mainly consist of synthetic SCSs and other novel SCSs. This is because aquaculture systems are sensitive to effluent quality, requiring the use of synthetic carbon sources which can provide stable carbon release rates, less DOC accumulation, and lower effluent color intensity. The main aspects of interest in relevant studies were the conventional performance indicators of denitrification, including nitrate concentration, nitrite, and ammonia accumulation; TOC; and microbial community composition. In certain studies, quality changes in aquatic products (e.g., fishes) reared in systems with and without denitrification were compared; however, significant differences were not observed between the experimental and control groups (Boley and Müller, 2005). 3.2 Agricultural subsurface drainage systems Agricultural subsurface drainage systems are a commonly used agricultural drainage method in which excess groundwater and surface water is removed through underground (subsurface) drainage pipes (Schipper et al., 2010). The controlled agricultural drainage enables the elimination of waterlogging and control of groundwater levels, which are beneficial to the prevention of soil swamping and salinization, creating favorable conditions for agricultural production. In most existing subsurface drainage systems in China, water is directly discharged into nearby water bodies, and has a deleterious impact on the ecological environment (Chun et al., 2009). Consequently, the installation of denitrification bioreactors prior to drainage discharge has become widely accepted. The use of corn cobs, corn stover, wheat straw, cardboard fibers, leaf litter composts, tree bark, wood chips, and almond shells as carbon sources have been reported in previous studies (Christianson et al., 2016, Chun et al., 2009). Camilo et al. (Krause Camilo, 2016) constructed a horizontal flow reactor filled with wheat straw and pine bark mulch for the removal of nitratenitrogen and the herbicide agent atrazine (ATR) from subsurface drainage water. At 21 ºC and a HRT of 0.43 d, the removal rates of nitrate-nitrogen and ATR were 30 g N·(m·d)−1 and 22 mg ATR·(m·d)−1, respectively. David et al. (David et al., 2016) performed a three-year evaluation of two wood chip bioreactors and found that nitrate-nitrogen removal requirements could be satisfied during year one and the early part of year two due to the adequate release of soluble carbon. However, as operating time increased, temperature became the primary limiting factor of the nitrate-nitrogen removal rate. In another study, Li et al. (He et al., 2018) utilized wood chips and fly ash in tandem for the simultaneous removal of nitrate-nitrogen and phosphate pollution from subsurface drainage water. They found that the nitrate-nitrogen removal efficiency changed significantly with HRT. However, changes in phosphate removal efficiency with HRT were not significant, and orthophosphate adsorption by fly ash was far less than the saturated capacity determined from a previous study. The use of biomass denitrification beds for the purification of agricultural subsurface drainage water represents the main practical application of SCS-based denitrification technologies. Research in this field is also relatively well-established and highly relevant to practical applications. SCSbased denitrification beds located at the end of agricultural subsurface drainage systems mainly utilize natural SCSs as fillers, with wood chips being one of the most commonly used materials. Given the heterogeneity in application locations and substantial variations in water quality and volume, relevant studies have focused on the influence of temperature and HRT on denitrification and the measures required to overcome denitrification inhibition. Other key research directions include the age of filling materials (Ghane et al., 2018), decomposition and degradation of fillers (Seres et al., 2018), and leaching characteristics of DOC (Abusallout and Hua, 2017). 3.3 Surface water The input of pollutants beyond the carrying capacity has led to the aggravation of surface water eutrophication. For large water bodies with low pollutant concentrations, SCS-based denitrification technologies are a promising method to remove excessive nitrate-nitrogen and bring less side effect to water body (Chang et al., 2016). By adopting pretreated corn cobs, rice straw, and rice hulls as SCSs, Feng et al. (Xie et al., 2017) compared the impact of different carbon sources and pretreatment methods on SCS-based denitrification and analyzed the differences in effluent nitrogen pollutant concentration and microbial communities. They found that SCSs pretreated with acid or alkali achieved higher denitrification rates and lower effluent concentrations of ammonia-nitrogen and nitrites. In another study, Feng et al. (Feng et al., 2019) developed solid-phase denitrification systems using alkalipretreated rice husks, pomelo peels, and durian peels as biodegradable carriers for the simultaneous nitrification and denitrification of ammonia-nitrogen-polluted wastewater. High nitrogen removal rates (0.56–0.68 mg NH4+-N·(L·h)-1) and the identification of multiple new aerobic denitrifiers were realized. Another typical application of SCS-based denitrification technologies is in the removal of nitrogen pollutants in constructed wetlands. Jia et al. (Jia et al., 2019) utilized agricultural wastes (wheat straw) as carbon sources for the removal of nitrogen pollutants in a constructed wetland. The average dissolved organic carbon release rate was 5.24 mg·(g·d)−1, and three months assessment revealed TN removal efficiencies of 66.75–93.67 %. The DOM generated from the various agricultural wastes mainly consisted of humic and fulvic acid-like compounds. In another study, Shen et al. prepared cornstarch/PCL blends for use as SCSs in constructed wetlands. They found that the average denitrification rate and nitrate removal efficiency were 0.069 kg·(m3·d)-1 and 98.23 % (25℃, 72 h HRT), respectively, and the major component of DOM was polysaccharides which mainly consisted of reducing sugar (Shen et al., 2015). Si et al. selected wheat straw, cotton, PBS, and newspaper as external carbon sources for the comparison of NO3--N and TN removal rates under low and high temperatures, and found that newspaper achieved the highest removal rates under all temperature conditions (Si et al., 2018). Subsequently, 16S rRNA metagenomic sequencing was employed to investigate the influence of different SCSs on the structure and function of bacterial communities. Liu et al. constructed a wetland using PBS as the SCS for the treatment of ammonianitrogen-polluted wastewater under aerated conditions (Liu et al., 2018c). They found that TN removal rates of up to 99 % could be achieved, and simultaneous nitrification and denitrification was the main microbial nitrogen removal pathway. In addition to investigating nitrate-nitrogen removal, studies with respect to surface water bodies, including constructed wetlands, have mainly focused on ammonia-nitrogen and TN removal efficiencies and the simultaneous nitrification and denitrification process in the presence of SCSs. Furthermore, the impact of different carbon sources, temperatures, and DO levels on removal efficiencies and the DOM characteristics and functional microorganisms in effluents have also been frequently studied. 3.4 Groundwater The pollution of groundwater by nitrate-nitrogen is extremely severe in China (Ma et al., 2012). With the application of nitrogen fertilizers and the haphazard discharge of domestic sewage, nitrogen pollutants migrate to groundwater through the infiltration of surface runoff. Consequently, the adoption of SCS-based denitrification technologies for groundwater purification has received considerable attention. Chu et al. used a PHBV and bamboo powder blend as a carbon source and biofilm carrier in a packed-bed reactor for nitrate removal in groundwater (pump to surface). They found that the reactors achieved a rapid start-up without external inocula, nitrate removal efficiencies of up to 87.4 %, and less adverse effects in terms of nitrite accumulation (0.5 mg/L) and DOC release (10.5 mg/L) (Chu and Wang, 2016). When Xie et al. utilized a PHA/cellulose blend as a slow-release carbon material for the removal of nitrates from groundwater, they found that the blend exhibited excellent nitrate removal efficiency and less adverse effects in terms of nitrite accumulation during stable operations (Xie et al., 2017). In another study, Ye et al. developed a PHBV and ceramsite based permeable reactive barrier system, which was used in packed-bed reactors for the treatment of groundwater polluted with nitrate-nitrogen (Ye et al., 2017). Results of a continuous experiment conducted over a 35 day period indicated that more than 95 % of nitrate-nitrogen was removed and a maximum denitrification rate of 241 mg N·(L·d)-1 was achieved. Furthermore, they found that shortening the HRT significantly reduced the release of DOC. Jin et al. constructed a sawdust/pyrite mixotrophic denitrification reactor and analyzed the influence of sawdust dosage and HRT on reactor performance for in situ groundwater remediation (Jin et al., 2019). They found that an overdosage of sawdust increased nitrite-nitrogen and ammonia-nitrogen accumulation, and increasing the HRT from 12 to 24 h did not significantly enhance removal efficiency. The main aspects of interest in studies related to SCS-based groundwater treatment are similar to those of ordinary wastewater treatment and include the influence of the nitrate-nitrogen load, HRT, temperature, and carbon source dose on denitrification efficiency. Effluent parameters of interest mainly include nitrogen pollutant concentration, DOC concentration, and microbial community structure. 3.5 Summary of SCSs applications Table 2 provides a summary of the applications of SCSs in different target water bodies. Table 2. Characteristics of studies on the application of SCSs in different target water bodies Table 2 illustrates that differences in the characteristics and treatment requirements of the various target water bodies result in different carbon sources, process characteristics, factors of interest, and effluent parameters. For ordinary wastewater treatment, which is primarily aimed towards pollutant removal, effluent quality indicators are the primary concern. Furthermore, operating temperature and HRT and the accumulation of byproducts (e.g., DOC, nitrite-nitrogen, and ammonia nitrogen) in the effluent should also be examined. Consequently, studies on the application of SCS-based denitrification to ordinary wastewater treatment have mainly focused on achieving optimal effluent quality through parameter control. For recirculating aquaculture systems, effluent quality requirements are more stringent and treatment costs are usually higher compared to ordinary wastewater treatment. Consequently, synthetic SCSs with a low likelihood of secondary pollution, rapid carbon release, and high denitrification efficiency are commonly used. The influence of temperature on denitrification efficiency was rarely investigated with respect to recirculating aquaculture systems because the temperature is typically maintained within a certain range for aquatic product survival; however, the aquatic product yield was a key indicator. For agricultural subsurface drainage systems, natural agricultural and forestry wastes are the most common primary carbon sources due to the adaptability to local conditions and cost requirements. Research in this area is relatively well-established, with a key issue being the selection of the appropriate HRT to address large fluctuations in the quality and volume of drainage water. Because these drainage systems are mainly located in the open, reducing the constraints imposed by low temperatures on denitrification efficiency is also a key concern. Other areas of interest include denitrification efficiency at low HRT, byproduct concentrations in the effluent, carbon source operating life, and the simultaneous removal of nitrogen and phosphorus. Surface water is characterized by low pollutant concentrations and high flow rates, limiting microorganism enrichment, the formation of an adequate supply of localized carbon sources, and the denitrification ability of the microorganisms. This problem can be effectively resolved through the adoption of SCS-based denitrification technologies. Most existing studies have focused on conventional influencing factors (e.g. type and quantity of the carbon source and nitrogen pollutant load) and effluent quality parameters. Given the geographical and environmental characteristics of surface water, many researchers have also explored the influence of DO on the performance of carbon sources in surface water denitrification. In groundwater, which is characterized by low concentrations of easily oxidizable organic carbon and low temperatures, denitrification rates under natural conditions are usually lower (Wu, 2002). The microbial growth induced by the dosing of nutrient solutions in groundwater leads to a reduction in the pores of the water-containing medium, which consequently results in blockages; hence, the adoption of SCS-based denitrification technologies (especially denitrification barriers) has provided a novel means of nutrient supply. Existing studies on groundwater have mainly focused on the influences of nitrate-nitrogen load, HRT, and carbon source dosage on denitrification efficiency. Furthermore, the functional genes and microbial community structures related to the denitrification process have also been explored in research on the various target water bodies. 4 Meta-analysis on synthetic SCS-based denitrification In order to better understand the effect of different SCSs and reaction conditions on the denitrification efficiency, we applied meta-analysis on the published literature in the area of solidphase denitrification in recent years. Since the denitrification rates of synthetic carbon sources are much larger than that of raw carbon sources, in this study, we focus on the application of synthetic carbon source on nitrate removal. The data came from published journal articles that presented nitrate removal rates from flowthrough, synthesized SCS-based denitrification bed or lab-scale column reactors with nitrate as the main target containment. We searched for literature in the database of Web of Science with synthesized carbon sources (i.e. PBS, PCL, PHA, PHB, PHBV and PLA) and denitrification. Literature without long-term stable operation and key experimental data were excluded. 23 papers published focusing on synthesized carbon source based solid phase denitrification in recent three years were finally analyzed in our study. The core measure of solid-phase denitrification in our study was denitrification rates (DR) in the units of nitrate removal per volume of bioreactor per time (gNL-1d-1), and necessary calculation were applied using other information from papers when DR was not given directly. The data required for meta-analysis in our study were the mean value of DR and the corresponding standard deviation (SD). Based on our former discussion and available data, we chose carbon source type (CS type), carbon source species (CS species), influent N concentration, HRT and water temperature as target factors, which were further categorized into two or three levels, adapted from the meta analysis study on woodchip denitrification reactors (Addy et al., 2016). CS type is categorized into ‘mix CS’ (mixed synthetic carbon source or the mixture of synthetic and raw carbon sources) and ‘single CS’; three kinds of synthetic carbon source (i.e. PHBV, PHBV/PLA, PCL) with abundant experimental data are analyzed; Influent N concentration is divided into low, intermediate and high categories split by 20 and 50 mgN/L; HRT and water temperature are categorized in similar manner, split by 2 h and 5 h, 22℃ and 25℃, respectively. The actual data on mean removal rate, SD, number of studies and the classification of each factors are listed in Table S1. The response ratio (lnR) and the response variance (VlnR) were calculated (Addy et al., 2016) and MetaWin 2.0 was used for the calculation of nitrate removal rate effect size and its standard deviation. Forest plots of the meta-analysis results were shown in Figure 2. Figure 2. Mean nitrate removal effect size and 95% bias-corrected confidence interval by different categories of (a) CS type, (b) CS species, (c) Influent N concentration, (d) HRT and (e) Temperature. Numbers labeled in the figures are the mean value of effect size, and n represent for the number of studies in meta-analysis. There was no significant difference in nitrate removal rates between mix carbon source and single carbon source (Fig 2a), and while species of CS (PHBV, PHBV/PLA and PCL) were decisive as shown in Fig 2b. The mixing of carbon sources is generally for two purposes, one is to minimize the cost while ensuring the denitrification efficiency, and the other is to treat multiple pollutants simultaneously or in stages (Jiang et al., 2020, Yang et al., 2020a, Yang et al., 2020c). The small difference in denitrification performance showed that mixing of SCSs under specific water bodies and specific environments is worth further exploration. Nitrate removal rates were significantly effected by influent N concentration, as shown in Fig 2c. Reactors with high influent N concentration (>50 mgN/L) obtained higher denitrification rates than those with intermediate (20-50 mgN/L) and low (<20 mgN/L) influent N concentration. Higher nitrate concentration always resulted in larger reaction rates, and higher nitrate removal rates could also be obtained unless the exceeding of maximum denitrification capability of the system (Jiang et al., 2020, Xu et al., 2018a). HRT with different levels also significantly influenced nitrate removal rates (Fig 2d). However, the results showed that higher HRT (>5h) achieved the inferior performance on nitrate removal, which is contradictory to the results in a certain literature with the consideration of HRT levels (Ding et al., 2020, Yi et al., 2020, Zhang et al., 2021). This is mainly because, in some studies, researchers set a relatively long HRT in order to achieve a stable low nitrate effluent concentration (Feng et al., 2020a, Han et al., 2018, Lan et al., 2020). In fact, synthetic carbon sources often have short lag time and brilliant carbon release efficiency, and in many cases, HRT of 2h is sufficient for the thorough removal of nitrate (Fang et al., 2020, Shen et al., 2020). The nitrate removal rate effect sizes under different temperature were shown in Fig 2e. Low temperature (<20℃) significantly affect nitrate removal, where less COD release, nitrite accumulation and shift of denitrifying genus were detected at low-temperature (Shen et al., 2020, Xu et al., 2019b). Nevertheless, the high and intermediate categories are not significantly different in nitrate removal. There are two main reasons for this. First, the research literature analyzed mainly conducted lab-scale column reactors around room temperature (around 25℃), which was reasonable for the treatment of RAS, municipal waste water and surface water; second, the effect of low temperature on enzyme activities and microbial community related to carbon hydrolysis and denitrification are more obvious when it is below 15℃ (Jiang et al., 2020, Shen et al., 2020). Therefore, realizing high-efficiency denitrification under low temperature conditions (generally groundwater) is still a topic worthy of continued research. Synthetic solid-phase carbon sources have good application prospects for solid phase nitrification. The optimization of operation parameters (especially HRT), the mixing of synthetic carbon sources for actual complex water bodies and the further design of low temperature-tolerated reactors are important considerations for future study. 5 Advantage and disadvantage summary The eutrophication of water bodies remains a serious problem in many parts of the world; hence, research on the removal of nitrate-nitrogen pollution is extensive. In particular, research has focused on SCSs due to their ease of transport, low tendency for secondary pollution, long service life, and ease of management. In existing research, target water bodies for SCS-based denitrification include wastewater (including aquaculture wastewater), agricultural subsurface drainage, surface water, and groundwater. Relevant research on practical applications in agricultural subsurface drainage systems is extensive and well-established, while studies on practical applications in other water bodies are relatively scarce. Although SCS-based denitrification technologies have received widespread attention, they also possess certain shortcomings. Natural SCSs are inexpensive, easily acquirable, and have a long operating life; however, their applications are often limited due to unstable carbon release rates, excessive DOC release, and increased color intensity during the early stages of denitrification. Synthetic SCSs are readily utilized by microorganisms due to their strong bioaffinities, have a low tendency to cause secondary pollution due to their simple composition, and exhibit rapid carbon release rates and high denitrification efficiencies. Synthetic SCSs are superior to natural SCSs in most applications; however, they are expensive, have limited carbon release rates, and their denitrification performance is strongly influenced by temperature. While these factors currently limit the broader application of synthetic SCSs, it is anticipated that ongoing research will successfully address these issues. In conclusion, despite the presence of certain limitations, SCSbased denitrification technologies show promise for applications in many fields due to their superior advantages. 6 Conclusion Solid-phase carbon sources have good application prospects for solid phase nitrification. Research with respect to wastewater treatment has mainly been focused on the removal efficiency of nitrogen pollutants and DOC accumulation in the effluent, while studies on recirculating aquaculture systems have focused on product yield and water quality parameters. Agricultural subsurface drainage system research was extensive, and focused on natural SCSs and the influence of temperature and HRT on denitrification efficiency. The primary aspect of surface water research was the influence of DO on denitrification efficiency, while studies on groundwater were mainly focused on the influence of nitrate-nitrogen load, HRT, and carbon source dosage on denitrification efficiency. The optimization of operation parameters (especially HRT), the hybrid application of synthetic carbon sources and the further design of low temperature-tolerated reactors are worthy of continued study. Acknowledgement This work was supported by the National Natural Science Foundation of China (Grant No. 51809195), Postdoctoral Science Foundation of China (No. 2018M642083) and National Water Pollution Control and Treatment Science and Technology Major Project of China (Nos. 2017ZX07204004 and 2017ZX07204002). References Abusallout, I. and Hua, G., 2017. Characterization of dissolved organic carbon leached from a woodchip bioreactor. Chemosphere. 183, 36-43. Addy, K., et al., 2016. Denitrifying Bioreactors for Nitrate Removal: A Meta-Analysis. Journal of Environmental Quality. 45(3), 873-881. Bao, W., et al., 2019. Generation, characterization, perniciousness, removal and reutilization of solids in aquaculture water: a review from the whole process perspective. Reviews in Aquaculture. 11(4), 1342-1366. Boley, A. and Müller, W.-R., 2005. Denitrification with polycaprolactone as solid substrate in a laboratory-scale recirculated aquaculture system. Water Science and Technology. 52(10-11), 495502. Boley, A., et al., 2000. Biodegradable polymers as solid substrate and biofilm carrier for denitrification in recirculated aquaculture systems. Aquac. Eng. 22(1-2), 75-85. Canfield, D. E., et al., 2010. The Evolution and Future of Earth's Nitrogen Cycle. Science. 330(6001), 192-196. Chang, J., et al., 2016. Remediation of nitrate-contaminated wastewater using denitrification biofilters with straws of ornamental flowers added as carbon source. Water Science and Technology. 74(2), 416-423. Chen, X., et al., 2013. Regional Control of Groundwater Nitrogen Contamination. Geological Science and Techology Information. 32(6), 130. Cheng, H.-Y., et al., 2020. Aerobic denitrification performance and nitrate removal pathway analysis of a novel fungus Fusarium solani RADF-77. Bioresour. Technol. 295, 122250. Christianson, L. E., et al., 2012. A practice-oriented review of woodchip bioreactors for subsurface agricultural drainage. Appl. Eng. Agric. 28(6), 861-874. Christianson, L. E., et al., 2016. Denitrifying bioreactor clogging potential during wastewater treatment. Water Res. 105, 147-156. Chu, L. and Wang, J., 2011a. Comparison of polyurethane foam and biodegradable polymer as carriers in moving bed biofilm reactor for treating wastewater with a low C/N ratio. Chemosphere. 83(1), 63-68. Chu, L. and Wang, J., 2011b. Nitrogen removal using biodegradable polymers as carbon source and biofilm carriers in a moving bed biofilm reactor. Chemical Engineering Journal. 170(1), 220225. Chu, L. and Wang, J., 2013. Denitrification performance and biofilm characteristics using biodegradable polymers PCL as carriers and carbon source. Chemosphere. 91(9), 1310-1316. Chu, L. and Wang, J., 2016. Denitrification of groundwater using PHBV blends in packed bed reactors and the microbial diversity. Chemosphere. 155, 463-470. Chun, J. A., et al., 2010. Estimation of flow and transport parameters for woodchip-based bioreactors: II. field-scale bioreactor. Biosystems Engineering. 105(1), 95-102. Chun, J. A., et al., 2009. Estimation of flow and transport parameters for woodchip-based bioreactors: I. laboratory-scale bioreactor. Biosystems Engineering. 104(3), 384-395. David, M. B., et al., 2016. Temperature and Substrate Control Woodchip Bioreactor Performance in Reducing Tile Nitrate Loads in East-Central Illinois. J. Environ. Qual. 45(3), 822829. Deng, Y.-L., et al., 2017. The impact of DO and salinity on microbial community in poly(butylene succinate) denitrification reactors for recirculating aquaculture system wastewater treatment. AMB Express. 7(1), 113. Ding, W., et al., 2020. Effective control of the carbon release of starch/polyvinyl alcohol based on a polyamide coating in solid-phase denitrification. Environmental Science-Water Research & Technology. 6(12), 3293-3305. dos Santos, A. J., et al., 2018. From Obtaining to Degradation of PHB: A Literature Review. Part II. Ingeniería y Ciencia. 14(27), 207-228. Duan, L. a., et al.,2016. Denitrification performance using biodegradable polymer as carbon source to treat nitrified swine wastwater. 2016 ASABE Annual International Meeting. St. Joseph, MI, ASABE: 1. Fan, Z.-x. and Wang, J.-l., 2009. Denitrification using polylactic acid as solid carbon source. Huan jing ke xue= Huanjing kexue. 30(8), 2315-2319. Fan, Z., et al., 2012. Biological nitrate removal using wheat straw and PLA as substrate. Environmental Technology. 33(21), 2369-2374. Fang, D., et al., 2020. Polymer substrate reshapes the microbial assemblage and metabolic patterns within a biofilm denitrification system. Chemical Engineering Journal. 387. Feng, L., et al., 2019. Nitrification and aerobic denitrification in solid phase denitrification systems with various biodegradable carriers for ammonium-contaminated water purification. Journal of Chemical Technology & Biotechnology. 94(11), 3569-3577. Feng, L., et al., 2020a. Response of denitrifying community, denitrification genes and antibiotic resistance genes to oxytetracycline stress in polycaprolactone supported solid-phase denitrification reactor. Bioresource Technology. 308. Feng, L., et al., 2020b. Response of denitrifying community, denitrification genes and antibiotic resistance genes to oxytetracycline stress in polycaprolactone supported solid-phase denitrification reactor. Bioresour. Technol. 308, 123274. Feng, Y., et al., 2013. New types of extra carbon sources for denitrification. Modern Chemical Industry. 33(10), 52-57. Forrest, A. K., et al., 2010. Effects of temperature and pretreatment conditions on mixed-acid fermentation of water hyacinths using a mixed culture of thermophilic microorganisms. Bioresource Technology. 101(19), 7510-7515. Ghane, E., et al., 2018. Carbon Quality of Four-Year-Old Woodchips in a Denitrification Bed Treating Agricultural Drainage Water. Trans. ASABE. 61(3), 995-1000. Gomez, M. A., et al., 2000. Influence of carbon source on nitrate removal of contaminated groundwater in a denitrifying submerged filter. J. Hazard. Mater. 80(1-3), 69-80. Guo, Y. D., et al., 2017. Effects of hydraulic retention time (HRT) on denitrification using waste activated sludge thermal hydrolysis liquid and acidogenic liquid as carbon sources. Bioresour. Technol. 224, 147-156. Gutierrez-Wing, M. T., et al., 2012. Evaluation of polyhydroxybutyrate as a carbon source for recirculating aquaculture water denitrification. Aquacultural Engineering. 51, 36-43. Haihong, Z., et al., 2006. Denitrification Using PBS as Carbon Source and Biofiim Supporter: Effect of pH. Chinese journal of environmental science. 27(2), 290-293. Han, F., et al., 2018. Performance, microbial community and fluorescent characteristic of microbial products in a solid-phase denitrification biofilm reactor for WWTP effluent treatment. Journal of Environmental Management. 227, 375-385. Hang, Q. Y., et al., 2016. Application of plant carbon source for denitrification by constructed wetland and bioreactor: review of recent development. Environmental Science and Pollution Research. 23(9), 8260-8274. He, S., et al., 2018. Effect of hydraulic retention time on nitrogen removal and functional gene quantity/transcription in biochar packed reactors at 5 degrees C: A control-strategy study. Bioresource Technology. 264, 400-405. Healy, M. G., et al., 2012. Nitrate removal rate, efficiency and pollution swapping potential of different organic carbon media in laboratory denitrification bioreactors. Ecological Engineering. 40, 198-209. Hocking, P. J., et al., 1996. Enzymatic degradation of single crystals of bacterial and synthetic poly(beta-hydroxybutyrate). Macromolecules. 29(7), 2472-2478. Honda, Y. and Osawa, Z., 2002. Microbial denitrification of wastewater using biodegradable polycaprolactone. Polymer Degradation and Stability. 76(2), 321-327. Hu, R., et al., 2019. Effects of carbon availability in a woody carbon source on its nitrate removal behavior in solid-phase denitrification. Journal of Environmental Management. 246, 832839. Jafari, S. J., et al., 2015. High-rate biological denitrification in the cyclic rotating-bed biological reactor: Effect of COD/NO3-, nitrate concentration and salinity and the phylogenetic analysis of denitrifiers. Bioresour. Technol. 197, 482-488. Ji, F., et al., 2017. Denitrification performance of solid-phase denitrification biofilter and biochemical characteristics along its height. Chinese Journal of Environmental Engineering. 11(3), 1347-1354. Jia, L., et al., 2019. Exploring Utilization of Recycled Agricultural Biomass in Constructed Wetlands: Characterization of the Driving Force for High-Rate Nitrogen Removal. Environ. Sci. Technol. 53(3), 1258-1268. Jiang, L., et al., 2020. Denitrification performance and microbial diversity using starchpolycaprolactone blends as external solid carbon source and biofilm carriers for advanced treatment. Chemosphere. 255. Jin, S., et al., 2019. Effect of sawdust dosage and hydraulic retention time (HRT) on nitrate removal in sawdust/pyrite mixotrophic denitrification (SPMD) systems. Environmental Science: Water Research & Technology. 5(2), 346-357. Kessler, F., et al., 2014. Biodegradation improvement of poly(3-hydroxy-butyrate) films by entomopathogenic fungi and UV-assisted surface functionalization. Journal of Photochemistry and Photobiology B-Biology. 130, 57-67. Kim, J. S., et al., 2016. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresource Technology. 199, 42-48. Krause Camilo, B., 2016. Bioreactor reduces atrazine and nitrate in tile drain waters. Ecol. Eng. 86, 269-278. Lan, Z., et al., 2020. Comparative analysis of denitrification performance, denitrifying community and functional genes to oxytetracycline exposure between single and hybrid biodegradable polymers supported solid-phase denitrification systems. Biodegradation. 31(4-6), 289-301. Li, H., et al., 2019. Porous solid carbon source-supported denitrification in simulated mariculture wastewater. Environmental Technology, 1-8. Li, J., et al., 2012. Denitrification Performance of a Packed Bed Reactor Using Solid Carbon Source. Journal of Agro-Environment Science. 31(6), 1230-1235. Li, P., et al., 2016. Tertiary nitrogen removal for municipal wastewater using a solid-phase denitrifying biofilter with polycaprolactone as the carbon source and filtration medium. Water Research. 93, 74-83. Li, R., et al., 2017. Nitrate removal efficiency of a mixotrophic denitrification wall for nitratepolluted groundwater in situ remediation. Ecol. Eng. 106, 523-531. Liang, J., et al., 2015. Investigation of biological denitrification using biodegradable polymers cascade mini ring as carbon source. Chinese Journal of Environmental Engineering. 9(2), 633-638. Liu, D., et al., 2018a. Poly(butylene succinate)/bamboo powder blends as solid-phase carbon source and biofilm carrier for denitrifying biofilters treating wastewater from recirculating aquaculture system. Scientific Reports. 8(1), 3289. Liu, D., et al., 2018b. Poly(butylene succinate)/bamboo powder blends as solid-phase carbon source and biofilm carrier for denitrifying biofilters treating wastewater from recirculating aquaculture system. Scientific Reports. 8. Liu, H., et al., 2018c. Microbial nitrogen removal of ammonia wastewater in poly (butylenes succinate)-based constructed wetland: effect of dissolved oxygen. Applied Microbiology and Biotechnology. 102(21), 9389-9398. Liu, Y., et al., 2019. Nitrogen removal in a combined aerobic granular sludge and solid-phase biological denitrification system: System evaluation and community structure. Bioresour. Technol. 288, 121504. Lopardo, C. R. and Urakawa, H., 2019. Performance and microbial diversity of bioreactors using polycaprolactone and polyhydroxyalkanoate as carbon source and biofilm carrier in a closed recirculating aquaculture system. Aquaculture International. 27(5), 1251-1268. Lu, T., et al., 2017. Denitrification Performance of a Denitrifier-Augmented Packed-Bed Bioreactor with Solid Carbon Source. Acta Scientiarum Naturalium Universitatis Pekinensis. 53(5), 957-963. Lucas, N., et al., 2008. Polymer biodegradation: Mechanisms and estimation techniques – A review. Chemosphere. 73(4), 429-442. Luo, G., et al., 2019. Comparison of nitrate-removal efficiency and bacterial properties using PCL and PHBV polymers as a carbon source to treat aquaculture water. Aquaculture and Fisheries. Luo, G., et al., 2016. Effect of dissolved oxygen on nitrate removal using polycaprolactone as an organic carbon source and biofilm carrier in fixed-film denitrifying reactors. Journal of Environmental Sciences. 43, 147-152. Ma, H., et al., 2012. Status of Nitrate Nitrogen Contamination of Groundwater in China. Journal of Soil Science. 43(6), 1532-1536. Mateju, V., et al., 1992. BIOLOGICAL WATER DENITRIFICATION - A REVIEW. Enzyme Microb. Technol. 14(3), 170-183. Muller, W. R., et al., 1992. Aspects of PHA (poly-B-hydroxy-butyric-acid) as an h-donator for denitrification in water treatment processes. Water Supply. 10, 79–90. Ovez, B., et al., 2006. Biological denitrification in drinking water using Glycyrrhiza glabra and Arunda donax as the carbon source. Process Biochemistry. 41(7), 1539-1544. Podduturi, R., et al., 2020. Geosmin fluctuations and potential hotspots for elevated levels in recirculated aquaculture system (RAS): A case study from pikeperch (Stizostedion lucioperca) production in Denmark. Aquaculture. 514, 734501. Qiu, T., et al., 2017. Bacterial community dynamics in a biodenitrification reactor packed with polylactic acid/poly (3-hydroxybutyrate-co-3-hydroxyvalerate) blend as the carbon source and biofilm carrier. Journal of Bioscience and Bioengineering. 123(5), 606-612. Rout, P. R., et al., 2017. Assessing Possible Applications of Waste Organic Solid Substances as Carbon Sources and Biofilm Substrates for Elimination of Nitrate Toxicity from Wastewater. Journal of Hazardous, Toxic, and Radioactive Waste. 21(3), 04016027. Ruan, Y.-J., et al., 2016. Simultaneous ammonia and nitrate removal in an airlift reactor using poly(butylene succinate) as carbon source and biofilm carrier. Bioresource Technology. 216, 10041013. Schipper, L. A., et al., 2010. Denitrifying bioreactors—An approach for reducing nitrate loads to receiving waters. Ecological Engineering. 36(11), 1532-1543. Seres, M., et al., 2018. The impact of woodchip-gravel mixture on the efficiency and toxicity of denitrification bioreactors. The Science of the total environment. 647, 888-894. Shah, A. A., et al., 2008. Biological degradation of plastics: A comprehensive review. Biotechnology Advances. 26(3), 246-265. Shen, Q., et al., 2020. The influence mechanism of temperature on solid phase denitrification based on denitrification performance, carbon balance, and microbial analysis. Science of the Total Environment. 732. Shen, Z., et al., 2013. Denitrification performance and microbial diversity in a packed-bed bioreactor using biodegradable polymer as carbon source and biofilm support. Journal of Hazardous Materials. 250-251, 431-438. Shen, Z., et al., 2015. Enhanced removal of nitrate using starch/PCL blends as solid carbon source in a constructed wetland. Bioresource Technology. 175, 239-244. Si, Z., et al., 2018. Intensified heterotrophic denitrification in constructed wetlands using four solid carbon sources: Denitrification efficiency and bacterial community structure. Bioresource Technology. 267, 416-425. Steidl, J., et al., 2019. Nitrogen retention efficiency of a surface-flow constructed wetland receiving tile drainage water: A case study from north-eastern Germany. Agriculture, Ecosystems & Environment. 283, 106577. Sun, G., et al., 2019a. Enhanced removal of nitrate and refractory organic pollutants from biotreated coking wastewater using corncobs as carbon sources and biofilm carriers. Chemosphere. 237, 124520. Sun, H., et al., 2019b. Simultaneous removal of nitrogen and pharmaceutical and personal care products from the effluent of waste water treatment plants using aerated solid-phase denitrification system. Bioresource Technology. 287, 121389. Sun, H., et al., 2020. Enhanced simultaneous nitrification and denitrification performance in a fixed-bed system packed with PHBV/PLA blends. International Biodeterioration & Biodegradation. 146, 104810. Takahashi, M., et al., 2011. Nitrate Removal Efficiency and Bacterial Community Dynamics in Denitrification Processes Using Poly (L-lactic acid) as the Solid Substrate. Microbes Environ. 26(3), 212-219. Wang, J. and Chu, L., 2016. Biological nitrate removal from water and wastewater by solidphase denitrification process. Biotechnology Advances. 34(6), 1103-1112. Wu, B., et al., 2021. Mechanism insights into polyhydroxyalkanoate-regulated denitrification from the perspective of pericytoplasmic nitrate reductase expression. Science of the Total Environment. 754. Wu, W., et al., 2012. Biological denitrification with a novel biodegradable polymer as carbon source and biofilm carrier. Bioresource Technology. 118, 136-140. Wu, W., et al., 2013. Denitrification performance and microbial diversity in a packed-bed bioreactor using PCL as carbon source and biofilm carrier. Applied Microbiology and Biotechnology. 97(6), 2725-2733. Wu, Y., 2002. Denitrification in groundwater systems. Techniques and equipment for environmental pollution control. 3(3), 27-31. Xie, Y., et al., 2017. Slowly released carbon source from composite materials system for removing nitrate pollution in groundwater. Rsc Advances. 7(17), 10215-10220. Xiong, R., et al., 2019. Biological denitrification using polycaprolactone-peanut shell as slowrelease carbon source treating drainage of municipal WWTP. Chemosphere. 235, 434-439. Xiong, R., et al., 2020. Comparison of agricultural wastes and synthetic macromolecules as solid carbon source in treating low carbon nitrogen wastewater. Science of the Total Environment. 739. Xu, Z., et al., 2018a. Effect of different carbon sources on denitrification performance, microbial community structure and denitrification genes. Science of the Total Environment. 634, 195-204. Xu, Z., et al., 2019a. Biological denitrification using PHBV polymer as solid carbon source and biofilm carrier. Biochemical Engineering Journal. 146, 186-193. Xu, Z., et al., 2019b. Effect of temperature on tertiary nitrogen removal from municipal wastewater in a PHBV/PLA-supported denitrification system. Environmental Science and Pollution Research. 26(26), 26893-26899. Xu, Z., et al., 2018b. PHBV polymer supported denitrification system efficiently treated high nitrate concentration wastewater: Denitrification performance, microbial community structure evolution and key denitrifying bacteria. Chemosphere. 197, 96-104. Xu, Z. X., et al., 2009. Biological Denitrification Using Corncobs as a Carbon Source and Biofilm Carrier. Water Environ. Res. 81(3), 242-247. Yang, F. and Wu, W., 2014. Biological denitrification using PHBV as carbon source and biofilm carrier. China Environmental Science. 34(7), 1703-1708. Yang, Z., et al., 2020a. Intensified simultaneous nitrification and denitrification performance in integrated packed bed bioreactors using PHBV with different dosing methods. Environmental Science and Pollution Research. 27(17), 21560-21569. Yang, Z., et al., 2020b. Nitrogen removal performance in pilot-scale solid-phase denitrification systems using novel biodegradable blends for treatment of waste water treatment plants effluent. Bioresource Technology, 122994. Yang, Z., et al., 2020c. Nitrogen removal performance in pilot-scale solid-phase denitrification systems using novel biodegradable blends for treatment of waste water treatment plants effluent. Bioresource Technology. 305. Yao, Z., et al., 2019. Development of a hybrid biofilm reactor for nitrate removal from surface water with macrophyte residues as carbon substrate. Ecol. Eng. 128, 1-8. Ye, L. T., et al., 2017. Denitrification of nitrate-contaminated groundwater in columns packed with PHBV and ceramsites for application as a permeable reactive barrier. Water Science and Technology-Water Supply. 17(5), 1241-1248. Yi, C., et al., 2020. Renovated filter filled with poly-3-hydroxybutyrateco-hydroxyvalerate and granular activated carbon for simultaneous removal of nitrate and PPCPs from the secondary effluent. Science of the Total Environment. 749. Zhang, H. W., et al., 2016a. Biological nitrate removal using a food waste-derived carbon source in synthetic wastewater and real sewage. J. Environ. Manage. 166, 407-413. Zhang, N., et al., 2016b. Growth, digestive enzyme activity and welfare of tilapia (Oreochromis niloticus) reared in a biofloc-based system with poly-beta-hydroxybutyric as a carbon source. Aquaculture. 464, 710-717. Zhang, Q., et al., 2016c. Effects of physicochemical properties of poly-epsilon-caprolactone on nitrate removal efficiency during solid-phase denitrification. Chemical Engineering Journal. 283, 604-613. Zhang, Q., et al., 2016d. Optimization of nitrate removal from wastewater with a low C/N ratio using solid-phase denitrification. Environmental Science and Pollution Research. 23(1), 698-708. Zhang, S., et al., 2021. Effect of filling ratio and backwash on performance of a continuousflow SPD reactor packed with PCL as carbon source. Water environment research : a research publication of the Water Environment Federation. Zhang, S., et al., 2018. Bioaugmentation with Diaphorobacter polyhydroxybutyrativorans to enhance nitrate removal in a poly (3-hydroxybutyrate-co-3-hydroxyvalerate)-supported denitrification reactor. Bioresource Technology. 263, 499-507. Zhang, S. S., et al., 2017. Heterotrophic nitrification and aerobic denitrification by Diaphorobacter polyhydroxybutyrativorans SL-205 using poly(3-hydroxybutyrate-co- 3hydroxyvalerate) as the sole carbon source. Bioresour. Technol. 241, 500-507. Zhang, Y., et al., 2014. Tracing nitrate pollution sources and transformation in surface- and ground-waters using environmental isotopes. Science of the Total Environment. 490, 213-222. Zhang, Z., et al., 2020. Recent advances in partial denitrification in biological nitrogen removal: From enrichment to application. Bioresource Technology. 298, 122444. Zhao, J., et al., 2020. Denitrification behavior in a woodchip-packed bioreactor with gradient filling for nitrate-contaminated water treatment. Biochemical Engineering Journal. 154, 107454. Zhenxing, F. and Jianlong, W., 2008. Denitrification at low temperatures using BDPs as the solid carbon source in a packed bed reactor. Journal of Tsinghua University. Science and Technology. 48(3), 436-439. Zhong, H., et al., 2020. Solid-phase denitrification for water remediation: processes, limitations, and new aspects. Critical Reviews in Biotechnology. 40(8), 1113-1130. Zhu, S.-M., et al., 2015. Biological denitrification using poly(butylene succinate) as carbon source and biofilm carrier for recirculating aquaculture system effluent treatment. Bioresource Technology. 192, 603-610. Table Click here to access/download;Table;Table1.docx Table 1. Summary of denitrification performance of different solid carbon source (SCSs) reported in previous literature Nitrate-nitrogen Denitrification concentration in Carbon source Scale Form rate HRT Note Reference untreated water [mg/(L·h)] (mg/L) Simultaneous removal of nitrate-nitrogen and A mix of wheat straw and pine bark mulch Pilot 100 1.25 0.43 d (Krause Camilo, 2016) the herbicide agent atrazine Macrophyte residues Laboratory Wood chips Laboratory Glycyrrhiza glabra and Arunda donax Laboratory 1.0–5.0 mm 5–8 2.12 50.04 1.49–7.27 (Yao et al., 2019) Gradient filling (Zhao et al., 2020) 0.29 100 (Ovez et al., 2006) 0.18 Corn cobs Laboratory Approx. 2 cm 24.5–25.5 8.46 153 L/d (Xu et al., 2009) 1.22 Pretreated sawdust 100 Laboratory Pretreated with (Hu et al., 2019) 1.25 peracetic acid Bioaugmentation by PBS Laboratory Approx. 3 mm 15 28.04 0.5 h high-efficiency (Lu et al., 2017) denitrifying bacteria PCL Laboratory 2.5–3.5 mm 30 19.00 1.5 h (Ji et al., 2017) 15 8.57 1.5 h (Liang et al., 2015) H=12.5 mm, D=25 mm PCL Laboratory (shaped into cascade mini ring) PCL Laboratory PCL Laboratory Poly(3-hydroxybutyrate-co-3- 2 × 3 × 4 mm 200 30.3 5.5 h (Luo et al., 2016) 5h (Luo et al., 2019) 11.25 2–3 mm 81.1–132.75 Laboratory 7.92 hydroxyvalerate) (PHBV) 911 m2/m3 (Gutierrez-Wing et al., PHB Laboratory (specific surface 50 2012) area) PHBV Laboratory 4–6.5 mm 44.75–57.25 10.04 2.6 h (Ye et al., 2017) 15 32.08 0.5 h (Xu et al., 2019a) 15 27.90 0.5 h (Yang & Wu, 2014) 50 1.65 H=3 mm D=3 PHBV Laboratory mm H=0.32 cm, PHBV Laboratory D=0.31 cm PLA Laboratory H≈3.02 mm, (Fan & Wang, 2009) D=2.22–3.60 mm PHB Laboratory 1.49 m2/L 7–41 Specific surface area- PCL Laboratory 0.87 m2/L 21–166 related denitrification Bionolle1 Laboratory 1.22 m2/L 5–40 1.5–10 0.75–1.25 h (specific surface Bionolle2 rates: 5–28; 20–160; (Boley et al., 2000) 1.3–9; 10.5–67 Laboratory 12–77 mg/(m2·h) area) H=5 mm D=3 PBS Laboratory Approx. 50 22.08 8h (Zhu et al., 2015) 50 3.64 12 h (Haihong et al., 2006) 15–18 5–7.5 2–3 h (Chu & Wang, 2016) Approx. 100 28.33–34.58 mm PBS Laboratory 1 × 2 × 3 mm H=3.5 mm PHBV/bamboo powder Laboratory D=2.5 mm PBS/BP Laboratory H=10 mm D=10 Freshwater/seawater (Liu et al., 2018a) mm (Shen et Starch/PCL Laboratory 3~5 mm 50 2.88 al., 2015) PHBV/PLA Laboratory 2.5–3 mm PLA/PHBV Laboratory 2.5–3 mm 15.42 (Xu et al., 2018a) 85 31.28 2.5 h (Qiu et al., 2017) 100 40.53 2h (Li et al., 2012) Oval-shaped particles with a PLA/PHBV Laboratory specific surface area of 0.015 m2/g Starch/polyolefins Laboratory 1 × 2 × 3 mm 60–80 2.5–4.5 Low temperatures (8– (Zhenxing & Jianlong, 10 ℃) 2008) 2.3–3.3 h Table Click here to access/download;Table;table2.docx Agricultural Target water body Wastewater Recirculating aquaculture systems subsurface drainage Surface water Groundwater systems (Duan et al., 2016; Rout et al., 2017; (Boley & Müller, 2005; Deng et al., 2017; Li (Abusallout & Hua, Shen et al., 2013; Sun (Feng et al., 2017; et al., 2019; Liu et al., 2018a; Luo et al., Nitrates 2017; David et al., et al., 2019a; Sun et Jin et al., 2019; Xie et Shen et al., 2015; 2019; Luo et al., 2016; Ruan et al., 2016; 2016; Krause Camilo, Zhu et al., 2015), 2016; Li et al., 2018) al., 2019b; Sun et al., Target pollutants (Chu & Wang, 2016; al., 2017; Ye et al., Yao et al., 2019) 2017) 2020; Xu et al., 2019b), Ammonia- (Sun et al., 2020; Xu (Feng et al., 2019; (Ruan et al., 2016) nitrogen et al., 2019b) Liu et al., 2018b), total nitrogen (Honda & Osawa, (Jia et al., 2019) 2002; Yang et al., 2020) Other refractory (Sun et al., 2019a; organic Sun et al., 2019b) substances Phosphates (Li et al., 2018), Herbicides (Krause Camilo, 2016) (Rout et al., 2017; (Abusallout & Hua, (Feng et al., 2017; Sun et al., 2019a), 2017; David et al., Jia et al., 2019; Yao Natural (Jin et al., 2019), Types of carbon natural blends (Rout 2016), (Krause et al., 2019) (Feng et sources et al., 2017) Camilo, 2016), al., 2019) (Duan et al., 2016; (Boley & Müller, 2005; Deng et al., 2017; Honda & Osawa, Luo et al., 2019; Luo et al., 2016; Ruan et Synthetic (Liu et al., 2018b), 2002; Sun et al., al., 2016; Zhu et al., 2015) 2019b; Xu et al., 2018b; Yang et al., 2020), (Sun et al., 2020; Xu (Chu & Wang, 2016; Ye et al., 2019b) et al., 2017) (Shen et al., 2013; (Chu & Wang, 2016; Synthetic blends Natural and (Liu et al., 2018a) synthetic blends (Shen et al., 2015) Yang et al., 2020) Xie et al., 2017) Natural blends synthetic with (Li et al., 2019) additives Natural in Li et al., 2018) tandem with other solid wastes (Abusallout & Hua, (Feng et al., 2017; 2017; David et al., Feng et al., 2019; Jia Fragmented (Rout et al., 2017; agricultural and (Jin et al., 2019), Sun et al., 2019a) 2016; Krause Camilo, et al., 2019; Yao et 2016; Li et al., 2018) al., 2019) forestry wastes (Duan et al., 2016; Forms of carbon Honda & Osawa, sources (Boley & Müller, 2005; Deng et al., 2017; 2002; Shen et al., (Chu & Wang, 2016; Liu et al., 2018a; Luo et al., 2019; Luo et al., Granules 2013; Sun et al., (Li et al., 2018) 2016; Ruan et al., 2016; Zhu et al., 2015), 2019b; Sun et al., 2019b; Xu et al., Xie et al., 2017; Ye et Shen et al., 2015) al., 2017) spherical gel (Li et al., 2019) 2020; Xu et al., (Liu et al., 2018b; 2018b; Yang et al., 2020) Batch (Rout et al., 2017) experiments (Honda & Osawa, 2002; Shen et al., 2013; Sun et al., Process Up flow fixed2019a; Sun et al., characteristics (Boley & Müller, 2005; Deng et al., 2017; Li (Feng et al., 2017; bed (Chu & Wang, 2016; 2019b; Sun et al., et al., 2019; Liu et al., 2018a; Luo et al., Feng et al., 2019; denitrification Jin et al., 2019) 2020; Xu et al., reactors 2019b; Xu et al., 2018b; Yang et al., 2020) 2019; Luo et al., 2016; Zhu et al., 2015), Yao et al., 2019) Airlift inner-loop (Ruan et al., 2016) reactors Horizontal flow (Abusallout & Hua, fixed-bed 2017; David et al., (Xie et al., 2017) denitrification 2016; Krause Camilo, reactors 2016; Li et al., 2018) (Jia et al., 2019; Liu Constructed et al., 2018b; Shen wetlands et al., 2015) Ractive barriers Nitrate (Xie et al., 2017) (Rout et al., 2017; Influencing factors (Zhu et al., 2015), concentration Shen et al., 2013) HRT (Rout et al., 2017; Xu (Xie et al., 2017), of interest (Deng et al., 2017) (Li et al., 2018) (Jin et al., 2019; Ye et et al., 2018b), al., 2017) (Shen et al., 2013; Xu (David et al., 2016; et al., 2019b) Krause Camilo, 2016) Temperature Initial pH (Shen et al., 2013), (Feng et al., 2019; DO (Deng et al., 2017; Luo et al., 2016) Liu et al., 2018b; Yao et al., 2019), Deng et al., 2017; Liu et al., 2018a; Zhu et salinity al., 2015) Feng et al., 2017; Type of carbon (Duan et al., 2016; (Jin et al., 2019; Ye et (Li et al., 2019; Luo et al., 2019) source Feng et al., 2019; Jia Yang et al., 2020), al., 2017) et al., 2019) Pretreatment of (Sun et al., 2019a) (Feng et al., 2017) carbon sources (Duan et al., 2016; Rout et al., 2017; Shen et al., 2013; Sun (Chu & Wang, 2016; (Boley & Müller, 2005; Li et al., 2019; Luo Nitrate concentration Jin et al., 2019; Xie et Shen et al., 2015; al., 2017; Ye et al., 2020; Xu et al., interest Krause Camilo, 2016; al., 2019b; Sun et al., 2016), parameters of (Feng et al., 2017; et al., 2019a; Sun et et al., 2019; Luo et al., 2016; Ruan et al., Effluent (David et al., 2016; Li et al., 2018) Yao et al., 2019), , 2017), , 2019b; Xu et al., 2018b) Denitrification (Li et al., 2019; Liu et al., 2018a; Zhu et al., rate 2015) (Duan et al., 2016; Nitrite and (Chu & Wang, 2016; Shen et al., 2013; Sun (Liu et al., 2018a; Ruan et al., 2016; Zhu et (Feng et al., 2017) ammonia- Jin et al., 2019; Xie et et al., 2019b; Sun et al., 2015), (Liu et al., 2018a; Luo et al., (Feng et al., 2017; nitrogen al., 2017; Ye et al., al., 2020; Yang et al., 2019; Ruan et al., 2016; Zhu et al., 2015) Feng et al., 2019) concentration 2017), (Jin et al., 2019) 2020) (Honda & Osawa, (Feng et al., 2019; 2002; Sun et al., TN concentration (Luo et al., 2016), Jia et al., 2019; Liu 2020; Yang et al., et al., 2018b) 2020), (Duan et al., 2016; (Jia et al., 2019; TOC Shen et al., 2013; Sun (Liu et al., 2018a; Ruan et al., 2016; Zhu et (Abusallout & Hua, (Chu & Wang, 2016; Shen et al., 2015; accumulation et al., 2020; Xu et al., al., 2015), 2017) Ye et al., 2017) Yao et al., 2019) 2019b; Yang et al., 2020) Changes in mass (Honda & Osawa, and form of 2002; Xu et al., carbon source 2018b) Eemoval rates of (Li et al., 2018), (Jin et al., 2019), , (Jin (Krause Camilo, 2016) et al., 2019) (Sun et al., 2019b) other pollutants Material (Li et al., 2019; Zhu et al., 2015) (Boley & characteristics, Müller, 2005) (Boley & Müller, 2005) (Deng pH changes, et al., 2017) weight gain in (Jin et al., 2019) fish, microbial community structures cidovorax (Duan et Acidovorax (Feng et al., 2016; Shen et al., al., 2017), Acidovorax (Xie et al., 2013), Bacillus (Shen et al., 2017), Bacillus (Rout et al., Acidovorax (Luo et al., 2019), Azoarcus 2015), Azospira (Xie et al., 2017), (Ruan et al., 2016), Bdellovibrio (Luo et al., Bosea (Feng et al., 2017), Crotonatovorans Comamonas (Duan et 2019), Denitratisoma (Luo et al., 2019), 2017), (Chu & Wang, 2016), al., 2016), Simplicispira (Ruan et al., 2016) Dechloromonas Firmicus (Chu & Wang, Dechloromonas (Feng et al., 2017), 2016), Rhizomicrobium (Duan et al., 2016), Thauera (Shen et al., (Xie et al., 2017) Diaphorobacter (Shen 2015), Simplicispira Microorganisms et al., 2013), Ochrobactrum (Rout et al., 2017), Stenotrophomonas (Rout et al., 2017) (Feng et al., 2017) Figure Click here to access/download;Figure;1.tif Click here to access/download;Figure;Figure 2.tif Click here to access/download Supplementary material for on-line publication only Supplementary Information.docx *Declaration of Interest Statement Declaration of interests ☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. ☐The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: