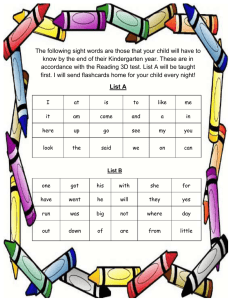
" ! STUDYLIB DOCUMENTS FLASHCARDS CHROME EXTENSION ≡ ' Login 1984 AP Chemistry Practice MC ONLY Exam COMPLETE ( Flashcards ) Collections & Download A) Cu2+ + SO42– + 2 NH4+ + 2 OH– → (NH4)2SO4 + Cu(OH)2 B) Cu2+ + 4 NH3 + 4 H2O → Cu(OH)42– + 4 NH4+ C) Cu2+ + 2 NH3 + 2 H2O → Cu(OH)2 + 2 NH4+ D) Cu2+ + 4 NH3 → Cu(NH3)42+ * Documents Cu2+ + 2 NH3 + H2O → CuO + 2 NH4+ E) 82. + Profile Step 1 N2H2O2 p N2HO2– + H+ (fast equilibrium) Step 2 N2HO2– → N2O + OH– (slow) Step 3 H+ + OH– → H2O (fast) Nitramide, N2H2O2, decomposes slowly in aqueous solution. This decomposition is believed to occur according to the reaction mechanism above. The rate law for the decomposition of nitramide that is consistent with this mechanism is given by which of the following? A) B) C) D) E) Rate = k [N2H2O2] Rate = k [N2H2O2] [H+] Rate = (k [N2H2O2]) / [H+] Rate = (k [N2H2O2]) / [N2HO2–] Rate = k [N2H2O2] [OH–] 83. 5 O (g) p H2NCH2COOH(s) + 3 H2O(l) 2 2 At constant temperature, Δ H, the change in enthalpy for the reaction above is approximately equal to NH3(g) + 2 CH4(g) + A) Δ E – 11 RT 2 Page: 16 of 40 Is the category for this document correct? Automatic Zoom # $ × % Science / Chemistry No more boring flashcards learning! Learn languages, math, history, economics, chemistry and more with free Studylib Extension! ■Distribute all flashcards reviewing into small sessions ■Get inspired with a daily photo ■Import sets from Anki, Quizlet, etc ■Add Active Recall to your learning and get higher grades! Add to Chrome It's free Related documents Research Abstract Chem 31 Formal Lab Report… Check List Products Support Make a suggestion Documents Report Flashcards Partners Did you find mistakes in interface or texts? Or do you know how to improve StudyLib UI? Feel free to send suggestions. Its very important for us! Extension Examplum - Context Dictionary © 2013 - 2022 studylib.net all other trademarks and copyrights are the property of their respective owners DMCA Privacy Terms Send feedback