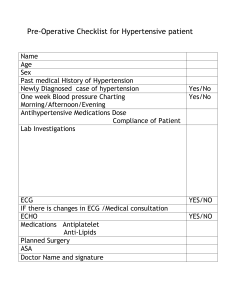
Presenter Essay question: Discuss the likely post-mortem findings in a 65 years old man who had been known to suffering from essential hypertension for the past 15 years. Answers: Introduction: Essential hypertension is idiopathic (causes of hypertension largely unknown – multifactorial: genetic and environmental factors), accounts for 90 – 95 % of all cases of hypertension. Patient who had been known to suffer from essential hypertension can develop complications in the heart, brain and kidneys that can leads to death. Besides increasing risk of atherosclerosis, hypertension can cause cardiac hypertrophy, and heart failure (hypertensive heart disease), multi-infarct dimentia, aortic dissection and renal failure. The prevalence and vulnerability to complications of hypertension increase with age (especially more than 60 years old) and are higher among Africans Americans. Unfortunateley hypertension typically remains asymptomatic until late in its course, and even severely elevated pressures can be clinically silent for years. Left untreated, roughly half of hypertensive patients die of ischemic heart disease or congestive heart failure, and another third die of stroke. Complications of hypertension: 1. Hypertensive vascular disease: i. Small vessel disease ii. Atherosclerosis iii. Aortic dissection 2. Hypertensive heart disease: i. Cardiac hypertrophy ii. Heart failure 3. Hypertensive nephropathy i. Nephrosclerosis ii. Renal failure Post-mortem findings: 1. Hypertensive vascular disease: Hyptertension leads to a number of vessel wall abnormalities, including atherosclerosis in larger arteries, hyaline arteriosclerosis in smaller arteries and (in severe cases) proliferative changes and frank necrosis that leads to rupture of arterioles. i. Small vessel disease : a) Hyaline arteriolosclerosis Fig 1: The arteriolar wall is thickened with increased protein deposition (hyalinized), and the lumen is markedly narrowed. b) Hyperplastic arteriosclerosis Fig 2: Hyperplastic arteriolosclerosis (onionskinning) causing luminal obliteration (arrow) ii. Atherosclerosis: Pathogenesis: Chronic endothelial injury and endothelial dysfunction cause intimal lesions called fatty streak, then matured into atheromas (also called atheromatous or atherosclerotic plaques). This produce stenosis of the blood vessels. The most involved vessels are (descending order): 1) Lower abdominal aorta 2) Iliac arteries 3) Coronary arteries 4) Internal carotid arteries and 5) vessels of the Circles of Willis. Fig 3: Aorta with fatty streaks (arrow), a collection of foamy macrophages in the intima associated largely with the ostia of branch vessels. Fig 4: Microscopic view of fatty streak demonstrating intimal, macrophagederived foam cells (arrow) Fig 5: Mild atherosclerosis in the aorta composed of fibrous plaques Fig 6: Severe atherosclerosis with diffuse, complicated lesions including an ulcerated plaque (open arrow) and a lesion with overlying thrombus. C A B Fig 8: Histologic features of atheromatous plaque in the coronary artery. (A) Overall architecture demonstrating fibrous cap (F) and a central necrotic core (C) containing cholesterol and other lipids. The lumen (L) has been moderately compromised. Note that a segment of the wall is plaque-free (arrow); the lesion is therefore “eccentric.” In this section, collagen has been stained blue (Masson trichrome stain). (B) Higher power photograph of a section of the plaque shown in (A), stained for elastin (black), demonstrating that the internal and external elastic membranes are attenuated and the media of the artery is thinned under the most advanced plaque (arrow). (C) Higher magnification photomicrograph at the junction of the fibrous cap and core showing scattered inflammatory cells, calcification (arrowhead), and neovascularization (small arrows). D E Fig. 9: Atherosclerotic plaque rupture. (D) Plaque rupture without superimposed thrombus in a patient who died suddenly. (E) Acute coronary thrombosis superimposed on an atherosclerotic plaque with focal disruption of the fibrous cap, triggering fatal myocardial infarction. In both (A) and (B), an arrow points to the site of plaque rupture. Atherosclerotic plaque can undergo important pathologic changes, including: 1. Rupture, ulceration and erosion of the surface of atherosclerotic plaque can lead to thrombosis, which can partially or completeley occlude the vessel lumen. 2. Thrombotic plaque rupture and embolise into bloodstream, producing atheroembolism. 3. Aneurysm formation due to degenerative changes and ischemic atrophy of the underlying tunica media with loss of elastic tissue, causes weakness and potential rupture. 4. Dissection and rupture of aneurysm. Most commonly in the abdominal aorta and common iliac arteries. As a consequence, atherosclerosis can cause major events such as myocardial infarction (heart attack), cerebral infarction (stroke), aortic aneurysms and aortic dissection that can be the cause of death in this patient. Fig. 10: Abdominal aortic aneurysm. (A) External view, gross photograph of a large aortic aneurysm that ruptured (rupture site is indicated by the arrow). (B) Opened view, with the location of the rupture tract indicated by a probe.The wall of the aneurysm is exceedingly thin, and the lumen is filled by a large quantity of layered but largely unorganized thrombus. 2. Hypertensive heart disease: Hypertensive heart disease is a consequence of the increased demands places on the heart by hypertension, causing pressure overload and ventricular hypertrophy. High blood pressure (hypertension) leads to the heart muscle pumping against increased resistance, which causes marked thickening of the left ventricular wall. The heart weight may exceeds 500 g, and the left ventricle wall thickness may exceed 2.0 cm. Dilatation of the ventricular chamber, thinning of the walls and enlargement of the external dimensions of the heart occur with the onset of decompensation (heart failure). Fig. 11: Left ventricular hypertrophy. (A) Pressure hypertrophy due to left ventricular outflow obstruction. The left ventricle is on the lower right in this apical four-chamber view of the heart. (B) Left ventricular hypertrophy with and without dilation, viewed in transverse heart sections. Compared with a normal heart (center), the pressure-hypertrophied hearts (left and in A) have increased mass and a thick left ventricular wall, and the hypertrophied, dilated heart (right) has increased mass and an apparently normal wall thickness. Fig. 12: Hypertensive heart disease, systemic and pulmonary. (A) Systemic (left-sided) hypertensive heart disease. There is marked concentric thickening of the left ventricular wall causing reduction in lumen size.The left ventricle and left atrium (asterisk) are on the right in this apical four-chamber view of the heart. A pacemaker is present in the right ventricle (arrow). (B) Pulmonary (right-sided) hypertensive heart disease (cor pulmonale). The right ventricle is markedly dilated and has a thickened free wall and hypertrophied trabeculae (apical four-chamber view of heart, right ventricle on left). The shape of the left ventricle (to the right) has been distorted by the enlarged right ventricle. Fig. 13: Microscopic findings of (C) Normal myocardium and (D) Hypertrophied myocardium (C and D are photomicrographs at the same magnification). Note the increases in both cell size and nuclear size in the hypertrophied myocytes, and the interstitial cells remain small. 3. Hypertensive complications affecting the brain: Hypertension is an important risk factor for brain infarction and haemorrhage (stroke) which can cause death. This result from two mechanism: i. ii. iii. Ischemia and/or hypoxia, from thrombotic occlusion of cerebral arteries by ruptured atherosclerotic plaque or thromboemboli. Hemorrhage (intraparenchymal), from rupture of CNS vessels induced by hypertension, most commonly occur in basal ganglia and thalamus designated as “ganglionic hemorrhage”. Lacunar infarct, develop from arteriosclerosis (small vessel disease) that progress to thrombosis and complete vessel occlusion of deep penetrating arterioles that supply the basal ganglia, hemispheric white matter and brainstem. Fig.(A) Massive hypertensive ganglionic hemorrhage rupturing into a lateral ventricle. Fig.(B) Hyaline arteriolosclerosis (fibrosis and thickening of the arteriolar walls) develops in the basal ganglia and subcortical white matter of patients with long-standing hypertension; it is a risk factor for hypertensive hemorrhages as well as lacunar infarcts. C D Fig.(C) Lacunar infarcts in the caudate and putamen (basal ganglia). Fig.(D) Small cavitary infarct (lacunar infarct) on microscopic examination. The lesion is characterized by lakelike spaces (area of tissue loss) surrounded by gliosis. 3. Hypertensive nephropathy Chronic untreated hypertension also can cause nephrosclerosis. It is defined as sclerosis of renal arterioles and small arteries (or arteriolosclerosis / arteriosclerosis) which causes narrowing of the lumen, diffuse impairment of renal blood supply and consequent glomerular scarring. Pathophysiology: 1. Narrowed lumen of renal vasculature (arteries and arterioles) by: i. medial and intimal wall thickening, and ii. hyalinization 2. Focal parenchymal ischemia, that leads to: i. Glomerulosclerosis ii. Chronic tubulointerstitial injury, fibrosis and tubular atrophy; iii. Reduction in renal mass (due to cortical scarring and shrinking) Gross: Kidney reduced in size Average weights between 110 and 130 g. The cortical surfaces have a fine, even granularity that resembles grain leather Microscopic: Fig: Close-up of the gross appearance of the cortical surface in benign nephroscleros is illustrating the fine, leathery granularity of the surface. Hyaline atherosclerosis Foci of tubular atrophy and interstitial fibrosis A variety of glomerular alterations : collapse of the GBM, deposition of collagen within the Bowman space, periglomerular fibrosis, and total sclerosis of glomeruli. B A A. Nephrosclerosis. Fibrointimal proliferation of the arcuate artery (PAS, 150x) B. Hyaline arteriolosclerosis. High-power view of two arterioles with hyaline deposition, marked thickening of the walls, and a narrowed lumen. C D C. Foci of tubular atrophy (arrow), (PAS, 200x) D. Tubular atrophy and interstitial fibrosis. E E. Nephrosclerosis. The glomerular tuft is shrunken, with wrinkling of the capillary walls (asterisk), global glomerular sclerosis (arrow), (PAS, 250x).