Electronic Structure of First 20 Elements Worksheet
advertisement

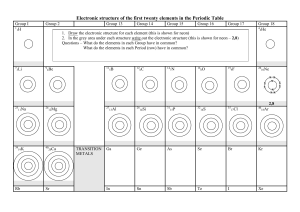
Electronic structure of the first twenty elements in the Periodic Table Group I 1 1H Group II 7 9 3Li Group III Group IV Group V Group VI Group VII 1. Draw the electronic structure for each element (this is shown for neon) 2. In the grey area under each structure write out the electronic structure (this is shown for neon – 2,8) Questions – What do the elements in each Group have in common? What do the elements in each Period (row) have in common? Draw and write out the electronic structure for a) a sodium ion b) a chloride ion 11 5B 4Be 12 6C 14 7N 16 8O 19 9F Group 0 4 2He 20 10Ne x x x x x x x x xx 2,8 23 11Na 24 12Mg 39 19K 40 20Ca Rb Sr TRANSITION METALS 27 13Al 28 14Si 31 15P 32 16S 35 17Cl 40 18Ar Ga Ge As Se Br Kr In Sn Sb Te I Xe