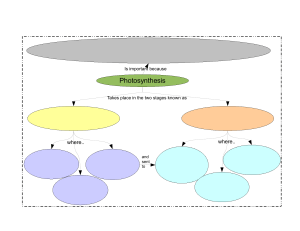
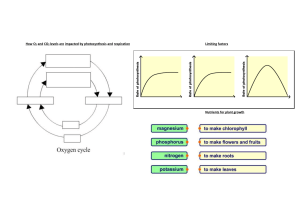
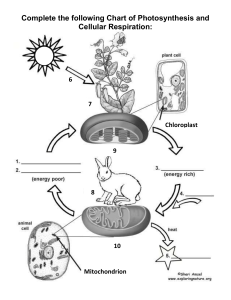
Cambridge IGCSE Laboratory Practical (ref: ISBN 9781444192193) Physics 1.0 Topic: General Physics 1.1 Simple pendulum 1.2 Density 1.3 Motion 1.4 Hooke’s Law 1.5 Balancing a beam 1.6 Centre of mass 1.7 Pressure 2.0 Topic: Thermal Physics 2.1 Specific heat capacity 2.2 Specific latent heat 2.3 Conduction and radiation 3.0 Topic: Properties of waves 3.1 Law of reflection 3.2 Refraction of light 3.3 Lenses 3.4 Speed of sound 4.0 Topic: Electricity and magnetism 4.1 Electric charges and currents 4.2 Resistance 4.3 Potential divider 4.4 Magnetism 4.5 Electromagnetism 4.6 Electric motor Topic: Atomic physics - Cambridge IGCSE Laboratory Practical (ref: ISBN 9781444192209) Chemistry Topic 1.0 The particulate nature of matter 1.1 Rate of diffusion of ammonia and hydrogen chloride (Teacher demonstration) 1.2 Reaction of potassium iodide with lead(II) nitrate 1.3 Sublimation of iodine Topic 2.0 Elements, compounds and experimental techniques 2.1 Rock salt; an important raw material 2.2 Ascending chromatography 2.3 Elements, mixtures and compounds (Part 1 Teacher demonstration) Topic 3.0 Atomic structure and bonding 3.1 Structure of substances 3.2 Properties of ionic and covalent substances 3.3 Electrolysis of solutions Topic 4 Stoichiometry- chemical calculations 4.1 Determination of the formula of magnesium oxide 4.2 Determination of the volume occupied by one mole of a gas 4.3 Determination of the percentage yield of a chemical reaction Topic 5 Electricity and chemistry 5.1 Electrolysis of lead(II) bromide (Teacher demonstration) 5.2 Electrolysis of water (Teacher demonstration) 5.3 Electrolysis of brine (Teacher demonstration) Topic 6 Chemical energetics 6.1 Electrochemical cells: chemical energy to electrical energy 6.2 Calculating the energy of combustion of methanol and ethanol 6.3 Determination of the energy change of a displacement reaction Topic 7 Chemical reactions 7.1 How does changing surface area affect the rate of a reaction? 7.2 What is the effect of changing the temperature on the rate of a reaction 7.3 What is the effect of changing the concentration on the rate of a reaction? Topic 8 Acids, bases and salts 8.1 Hydrated salts: how much water do they contain? 8.2 Determination of the concentration of a solution of hydrochloric acid 8.3 Preparation of hydrated magnesium sulfate Topic 9 The Periodic Table 9.1 Reactions of the Group 1 metals (Teacher demonstration) 9.2 Halogen displacement reactions 9.3 Using transition metal ions as catalysts Topic 10 Metals 10.1 Metal displacement reactions 10.2 Rusting of iron 10.3 Metal reactivity Topic 11 Air and water 11.1 The active part of the air (Teacher demonstration) 11.2 Making a fertiliser 11.3 The effects of add rain Topic 12 Sulfur 12.1 Sulfuric acid: a useful quantitative analytical chemical 12.2 Concentrated sulfuric acid (Teacher demonstration) 12.3 Properties of dilute sulfuric acid Topic 13 Inorganic carbon chemistry 13.1 Limestone: a useful resource 13.2 Does the food we eat contain carbon? 13.3 Carbon dioxide Topic 14 Organic chemistry 1 14.1 Is methane a hydrocarbon? 14.2 Difference between alkanes and alkenes 14.3 Hydrocarbons can form isomers Topic 15 Organic chemistry 2 15.1 Organic structures and functional groups 15.2 Nylon rope trick (Teacher demonstration) 15.3 Properties of dilute ethanoic acid Topic 16 Experimental chemistry 16.1 Missing labels from reagent bottles: what a problem! 16.2 Using flame colours to identify unknown metal ions 16.3 How pure is your water supply? Cambridge IGCSE Laboratory Practical (ref: ISBN 9781444191615) Biology Topic 1 Characteristics and classification of living organisms 1.1 Dichotomous keys Topic 2 Organisation and maintenance of the organism 2.1 Plant cells 2.2 Animal cells Topic 3 Movement in and out of cells 3.1 Osmosis and water flow 3.2 Turgor in potato tissue 3.3 Osmosis and turgor 3.4 Plasmolysis 3.5 Partial permeability (dialysis) Topic 4 Biological molecules 4.1 Food tests 4.2 Application of the food tests Topic 5 Enzymes 5.1 Extracting and testing an enzyme 5.2 The effect of temperature on an enzyme reaction 5.3 The effect of pH on an enzyme reaction Topic 6 Plant nutrition 6.1 Is chlorophyll necessary for photosynthesis? 6.2 Is light necessary for photosynthesis? 6.3 Is carbon dioxide needed for photosynthesis? 6.4 Is oxygen produced during photosynthesis? 6.5 The effect of light intensity on the rate of photosynthesis 6.6 Gaseous exchange during photosynthesis 6.7 The importance of different mineral elements Topic 7 Human nutrition 7.1 Energy from food Topic 8 Transport in plants 8.1 Transport in vascular bundles 8.2 Rates of water uptake in different conditions 8.3 To find out which surface of a leaf loses more water vapour Topic 9 Transport in animals 9.1 Physical activity and pulse rate Topic 11 Gas exchange in humans 11.1 Oxygen in exhaled air 11.2 Carbon dioxide in exhaled air 11.3 Volume of air in the lungs Topic 12 Respiration 12.1 Using up oxygen during respiration 12.2 Releasing energy in respiration 12.3 Anaerobic respiration in yeast Topic 14 Co-ordination and response 14.1 Gravitropism in pea radicles 14.2 Phototropism in shoots 14.3 Region of response Topic 16 Reproduction 16.1 Insect pollinated flowers 16.2 The growth of pollen tubes 16.3 Germination: The need for water 16.4 Temperature and germination Topic 20 Biotechnology and genetic Engineering 20.1 The effect of pectinase on fruit pulp