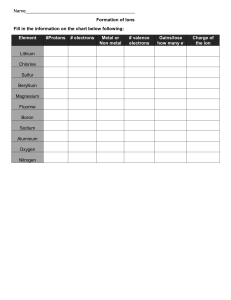
Valence electrons: electrons on the highest primary energy level of an atom and electrons outside of the atom - Max number of ve- is 8 All noble gasses have a full outer shell - Nobel gasses are unreactive and they hold on to their electron very tightly Fluorine is one of the most reactive atom cause its missing an electron - Fluorine will snatch electrons to fill in the missing electron - All atoms missing one electron from being stable are unstable Sodium has one extra electron and chlorine is missing one electron, so sodium will quickly throw its electron out and chlorine will quickly accept it - Therefore sodium becomes positive and chlorine becomes negative Octet rule: the tendency for atoms to obtain 8v e- This is why chem reactions occur - Helium is fine with 2 electrons tho How do atoms get 8 electrons - 1. Already have 8 [nobel gasses] - 2. Sharing electron [covalent bond] - ex) fluorine has 7 valence electrons - 2 fluorine atoms will group up with each other and form a diatomic element if each one can donate one electron to a shared pool of electrons - 3. Transferring electrons [ionic bond] - Na has 1 electron and cl has 7 electrons so sodium tries to transfer its outmost electron to chlorine so that chlorine can have 8 valence electrons. Because na lost a neg electron na becomes positive (cation) and because ch gained an electron it becomes negatively charged (anion). Determining the # of valance electrons - 1s2 2s2 2p6 3s2 4s2 3d10 4p3 - 1. Write out the electron configuration - 2. Identify the highest primary energy levels - 3. Count the valance electrons in these orbitals Oxygen: 1s2 2s2 2p4 = 8e & 6v eVertical row: group/family Horizontal row: row/series Everysingle group 2a has 2 valance electrons Lewis dot diagram: a visual representation of valence electrons using dots - Cation positively charged ions - More protons than electrons - The name is the same of the element it comes from - - Ions are positively or negatively charged - - Sodium cation More electrons than protons Anion are negatively charged ions - The name changes - Chlorine becomes chloride Atoms that have Atoms that have 1,2,3 valence electrons form +1,+2,+3, cations Atoms that have 5,6,7 valence electrons form -3,-2,-1, anions ex) mg 1s2,2s2,2p6,3s2 Formation of ionic bonds - Mg is trying to donate 2 valence electron but chlorine only has room for one, so mg will give it to 2 chlorines to form mgcl2 - IONIC COMPOUNDS ARE MADE FROM A METAL AND A NONMETAL (CATION & ANION) MG2O2 is the expectation and the real formula is MgO