Unit Conversions, Scientific Notation, and Molar Mass Worksheet
advertisement

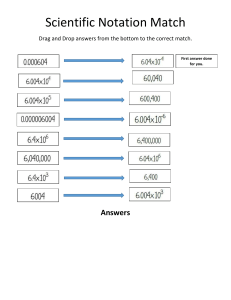
Unit analysis: Covert the following, don’t forget sig digs: 1.57kg to grams 654 µm to m 2hrs to hs 0.127 L to mL 0.78𝑘𝑚𝑜𝑙 𝑚𝑚𝑜𝑙 𝑡𝑜 𝑚𝑔 𝑔 0.786𝑔 𝑘𝑔 𝑡𝑜 𝑚𝐿 𝐿 Turn the following numbers into scientific notation: 0.000764 9765000000 Turn the following numbers from scientific notation to standard form: 5.76 x 10-5 2.34 x 108 30972 x 10 -8 Find the molar mass of the following compounds, keep your answers to 3 sig digs. H2O NH3 HSO4- Explain the difference(s) between Rutherford’s model and the Bohr model.