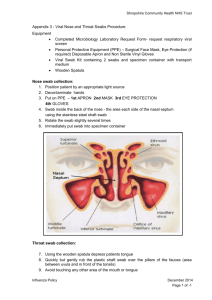
ALL INDIA INSTITUTE OF MEDICAL SCIENCES BIBINAGAR, TELANGANA - 508126 अखिलभारतीयआयुर्विज्ञानसंस्थान (एम्स) बीबीनगर, तेलंगाना – 508126, भारत Standard Operating Procedure for Rapid Antigen Testing for COVID-19 BACKGROUND Real time RT-PCR is the gold standard frontline test for diagnosis of COVID19. Various open and closed RT-PCR platforms (Open systems RT-PCR machines, TrueNat and CBNAAT) are currently being used for COVID19 diagnosis in India. All these platforms require specialized laboratory facilities in terms of equipment, biosafety & biosecurity. Minimum time taken for the test varies between different systems with a minimum of 2-5 hours including the time taken for sample transportation. These specifications limit the widespread use of the RT-PCR test and also impede quick augmentation of testing capacity in various containment zones and hospital settings. In view of this, there is urgent need of a reliable point-of-care rapid antigen detection test with good sensitivity and specificity for early detection of the disease. PURPOSE Rapid Antigen Detection Test for COVID-19 patients using the Standard Q COVID-19 Ag detection assay kit. PRINCIPLE AND METHOD OF THE PROCEDURE USED FOR EXAMINATION It is a rapid chromatographic immunoassay for qualitative detection of specific antigens to SARS-CoV-2. When the liquid sample is dropped on the sample pad, the antigen in the sample forms an immunocomplex with the antibody labeled with colloidal gold. Its complex moves along with the liquid sample, and makes a contact with the antibody immobilized on the membrane, followed by forming an immunocomplex with the immobilized antibody, resulting in generation of a colored red purple line. Appearance of red purple line on the membrane indicates the presence of antigen in the sample. Since the liquid of the sample migrates through the membrane very fast, it makes it possible to detect the presence or absence of antigen within 15 minutes. TARGET GROUP (according to ICMR guidelines): A. Routine surveillance in containment zones and screening at points of entry: Choice of Test (in order of priority): i. Rapid Antigen Test (RAT) [as per attached algorithm] ii. RT-PCR or TrueNat or CBNAAT 1. All symptomatic (ILI symptoms) cases including health care workers and frontline workers. 2. All asymptomatic direct and high-risk contacts (in family and workplace, elderly ≥ 65 years of age, immunocompromised, those with co-morbidities etc.) of a laboratory confirmed case to be tested once between day 5 and day 10 of coming into contact. 3. All asymptomatic high-risk individuals (elderly ≥ 65 years of age, those with co-morbidities etc.) in containment zones. *RAT for containment zone: Ideally, it is suggested that 100% people living in containment zones should be tested by RAT particularly in cities where there has been widespread transmission of infection. B. Routine surveillance in non-containment areas: Choice of Test (in order of priority): i. RT-PCR or TrueNat or CBNAAT ii. Rapid Antigen Test (RAT)* 4. All symptomatic (ILI symptoms) individuals with history of international travel in the last 14 days. 5. All symptomatic (ILI symptoms) contacts of a laboratory confirmed case. 6. All symptomatic (ILI symptoms) health care workers / frontline workers involved in containment and mitigation activities. 7. All symptomatic ILI cases among returnees and migrants within 7 days of illness. 8. *All asymptomatic high-risk contacts (contacts in family and workplace, elderly ≥ 65 years of age, those with co-morbidities etc. [RAT is recommended as the first choice of test in order of priority] C. In Hospital Settings: Choice of Test (in order of priority): i. RT-PCR or TrueNat or CBNAAT ii. Rapid Antigen Test (RAT) 9. All patients of Severe Acute Respiratory Infection (SARI). 10. All symptomatic (ILI symptoms) patients presenting in a healthcare setting. 11. Asymptomatic high-risk patients who are hospitalized or seeking immediate hospitalization such as immunocompromised individuals, patients diagnosed with malignant disease, transplant patients, patients with chronic co-morbidities, elderly ≥ 65 years. 12. Asymptomatic patients undergoing surgical / non-surgical invasive procedures (not to be tested more than once a week during hospital stay). 13. All pregnant women in/near labour who are hospitalized for delivery. Points to be noted: • No emergency procedure (including deliveries) should be delayed for lack of test. However, sample can be sent for testing if indicated as above (1-13), simultaneously. • Pregnant women should not be referred for a lack of testing facility. All arrangements should be made to collect and transfer samples to testing facilities. • Mothers who test positive for COVID-19 should be advised to wear a mask and undertake frequent handwashing while handling their baby for 14 days. They should also be advised on breast cleaning before feeding the neonate. These measures are likely to reduce transmission of COVID-19 to their babies. 14. All symptomatic neonates presenting with acute respiratory / sepsis like illness. (Features suggestive of acute respiratory illness in a neonate are respiratory distress or apnea with or without cough, with or without fever. Neonates may also manifest with only non-respiratory symptoms like fever, lethargy, poor feeding, seizures or diarrhea). 15. Patients presenting with atypical manifestations [stroke, encephalitis, hemoptysis, pulmonary embolism, acute coronary symptoms, Guillain Barre syndrome, Multiple Organ Dysfunction Syndrome, progressive gastrointestinal symptoms, Kawasaki Disease (in pediatric age group)] based on the discretion of the treating physician. D. Testing on demand (State Governments to decide simplified modalities): 16. All individuals undertaking travel to countries/Indian states mandating a negative COVID-19 test at point of entry. 17. All individuals who wish to get themselves tested. Tracking and contact tracing mechanisms should be ensured by the testing laboratories by notifying the public health authorities. PATIENT PREPARATION Not applicable. TIMINGS From 10 am to 1 pm from Monday to Saturday HEALTHCARE WORKER PREPARATION 1. Nasal swab and/or throat swab specimens is to be collected as per GoI guidelines. 2. Sample collection area should be kept clean and table or work surface should be properly disinfected with surgical spirit. 3. Collect required details from the patient and fill the sample collection form. 4. Clearly explain the sample collection procedure to ensure full cooperation from the patient. 5. Wear appropriate personal protective equipment (PPE) such as gloves, apron, N95 mask, head cap, goggles before starting the procedure. 6. Label the specimen collection vial containing VTM with the unique participant/ sample ID. 7. Specimen should be collected under good illumination. TYPE OF CONTAINER AND ADDITIVES • Each test kit comes with an inbuilt COVID antigen test device, viral extraction tube with viral lysis buffer and sterile swab for sample collection SPECIMEN REQUIREMENTS AND STORAGE OF SPECIMENS • One nasopharyngeal swab would be collected using the customized sample collection swab provided with the kit • Once the sample is collected in the extraction buffer, it is stable only for one hour. Therefore, the antigen test needs to be conducted at the site of sample collection in the healthcare setting. • Transportation to the lab is not recommended. SPECIMEN REJECTION CRITERIA • No other sample (throat swab, bronchoalveolar lavage or sputum) should be used. REAGENTS • Each test kit comes with an inbuilt COVID antigen test device (cassette), viral extraction tube with viral lysis buffer, nozzle cap and sterile swab for sample collection PREPARATION OF REAGENTS • Not applicable STORAGE AND STABILITY • The test kits should be stored between 2°C to 30° C, out of direct sunlight. • Kit materials are stable until the expiration date printed on the outer box. • Do not freeze the kit. EQUIPMENTS/MATERIALS • Standard Q COVID-19 Ag detection assay kit • Refrigerator • Droppers, if required NASAL SWAB/ NASOPHARYNGEAL SWAB COLLECTION Persons taking & location • Nasopharyngeal swab will be collected in one of the rooms situated behind the COVID-19 screening area near OPD entrance. • The patient would be standing outside and the technician inside, with a plastic screen separating both of them. • Microbiology technician would collect a nasopharyngeal swab (wearing the complete PPE kit) through one of the holes made within the plastic screen, thus avoiding direct contact with the patient. • Take a fresh sterile swab • Gently tilt the patient’s head backwards and steady the chin • Insert the swab into the nostril parallel (1-2 cm) to the palate until resistance is met at the turbinate • Hold the swab in that position for few seconds and then withdraw slowly in a firmly rotating motion (5 times clockwise and 5 times anticlockwise). • Appropriate precautions should be taken in collecting specimens since this may expose the lab technician/ sample collector to respiratory secretions from the patient. TEST PROCEDURE • Insert the swab into an extraction buffer tube. While squeezing the buffer tube, stir the swab more than 5 times • Remove the swab while squeezing the sides of the tube to extract liquid from the swab • Press the nozzle cap tightly onto the tube • Apply 2 drops of extracted specimen to the specimen well of cassette • Read the test result in 15-30 minutes. LABORATORY CLINICAL INTERPRETATION • The test can be interpreted as positive or negative after 15 minutes of putting the sample into the well by appearance of test and control lines, which can be read with a naked eye, requiring no specialized equipment. • The presence of test line (T) along with Control Line(C), no matter how faint the test line is, the result must be considered as positive. • The presence of band in the control line, without the presence of band in test line, indicates the test is Negative. • Absence of band in control line irrespective of test line indicates that the test is invalid. • Maximum duration for interpreting a positive or negative test is 30 minutes. • After that the test strip should be discarded. • Standard Q COVID-19 Ag rapid antigen detection test has a very high specificity in the range of 99.3 to 100%, while the sensitivity of the test is 50.6% to 84%. • A positive test by RAT would be declared positive, and does not need reconfirmation by RT-PCR test • All negative samples would be sent to the isolation centre (C block) located in the institute premises for RT-PCR testing. This centre is under the control of Telangana State Government via The District Medical & Health Officer, Bhuvanagiri District. LIMITATIONS AND/ INTERFERENCES • The antigen test can provide results in minutes; however, antigen tests may not detect all active infections, based on their mechanism of action. • These tests are very specific for the virus, but are not as sensitive as molecular PCR tests. • Therefore, positive results from antigen tests are highly accurate, but there is a higher chance of false negatives, so negative results do not rule out infection. • With this in mind, negative results from an antigen test may need to be confirmed with a PCR test (if patients are symptomatic) prior to making treatment decisions or to prevent the possible spread of virus due to a false negative. ENVIRONMENTAL AND SAFETY CONTROLS Biomedical waste disposal • Collect used PPEs such as goggles, face-shield, splash proof apron, Plastic Coverall, Hazmet suit, nitrile gloves into double layered RED bag. • Collect used masks (including triple layer mask, N95 mask, etc.), head cover/cap, shoe-cover, disposable linen Gown, non-plastic or semi-plastic coverall into double layered YELLOW bags. • Pre-treated viral transport media, plastic vials, vacutainers, eppendorf tubes, plastic cryovials, pipette tips should be disposed in RED bags. • PPEs used and other contaminated waste generated from patients or by COVID-19 waste handlers and pathologists shall be stored separately in YELLOW bag, and shall be pre-treated with Autoclaving / Microwaving before transfer to temporary storage area and then handed over to Common treatment Facility in YELLOW colour bags with specific marking as “COVID-19 Waste”. • All the Bio-medical waste would be handed over, everyday, to M/S Roma Enterprises (Bhuvanagiri) for final treatment and disposal. PROCUREMENT OF KITS The kits would be procured from District Medical and Health Officer (Bhuvanagiri Dt). PATIENT BENEFICIARIES Individuals from the surrounding towns of AIIMS, Bibinagar. ACCOUNTABILITY: Sample collection: Mr Hari Babu (lab technician) Performing the tests: Dr P Rajeshwari (Sr Resident) Reporting of results: Dr M Anuradha (Associate Professor) REFERENCES 1. ICMR guidelines- Advisory on Use of Rapid Antigen Detection Test for COVID-19 2. COVID-19 - Sample collection guidelines-Indian Council of Medical Research – National Institute of Epidemiology. 3. Standard Q antigen kit insert