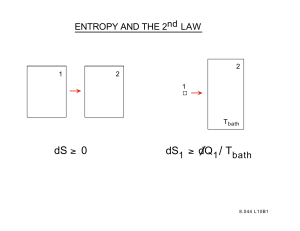
Activating Drugs with Sound- Mechanisms Behind Sonodynamic Therapy and the Role of Nanomedicine
advertisement

pubs.acs.org/bc Review Activating Drugs with Sound: Mechanisms Behind Sonodynamic Therapy and the Role of Nanomedicine 30th Anniversary Review Victor Choi,# Maneesha A. Rajora,# and Gang Zheng* Downloaded via UNIV OF TOKYO on April 12, 2020 at 12:35:28 (UTC). See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles. Cite This: https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 ACCESS Read Online Metrics & More Article Recommendations ABSTRACT: Sonodynamic therapy (SDT) is a promising therapeutic platform for minimally invasive cancer treatment in which acoustically susceptible drug agents, sonosensitizers, are activated by deep-tissue-penetrating low frequency ultrasound. Despite growing research in recent years, the field has yet to clearly elucidate broadly applicable mechanisms by which acoustic cavitation triggers sonosensitizer therapeutic activity, creating difficulties in achieving substantial and translatable therapeutic efficacy. In this review, we will critically analyze the proposed mechanisms underlying SDT and overview how nanomedicines can complement and extend these mechanisms to deliver more efficacious SDT. In doing so, we aim to highlight potential avenues toward viable implementation of SDT as a cancer therapy. 1. INTRODUCTION TO SONODYNAMIC THERAPY: A LOW FREQUENCY BIOMEDICAL APPLICATION OF ULTRASOUND The biomedical applications of ultrasound have evolved from its ubiquitous clinical use as an imaging tool to include an expanding array of therapeutic capabilities for the treatment of cancer. This arena of therapeutic ultrasound is rooted in the delivery of low (∼1 MHz)1 frequency pressure pulses to tissues at depths that can exceed 10 cm.2 These sound waves can be delivered in a focused manner, guided by imaging modalities such as magnetic resonance imaging (MRI), to target tissue. The energy deposited can translate into the generation of heat for tumor ablation, giving rise to high intensity focused ultrasound (HIFU)3 thermal ablation, or be pulsed at high intensities for nonthermal tissue fractionation via histotripsy,4 both of which serve as alternatives or adjuvants for conventional cancer treatment. Alternatively, at lower intensities, therapeutic ultrasound can generate largely nonthermal bioeffects when coadministered with microbubbles. These gas bubbles, stabilized by a lipid, polymer, or protein shell, are typically vascular agents that respond to the applied low frequency ultrasound by expanding and contracting. This phenomenon of microbubble oscillation,3 termed acoustic cavitation, has been widely exploited to enhance the extravasation and delivery of chemotherapeutic drugs to deep-seated target lesions, including beyond the blood-brain barrier to glioblastomas preclinically and clinically, as well as to pancreatic tumors, the liver and kidneys preclinically.5−10 © XXXX American Chemical Society Notably, the combined use of microbubble cavitation and a focused nonionizing energy source has made focused low intensity therapeutic ultrasound an attractive modality to enhance chemotherapy delivery to deep tissues in a safe, minimally invasive, controlled, and targeted manner.11 A less explored, but promising therapeutic application of low frequency, low intensity therapeutic ultrasound is the acoustic activation of drug agents, termed sonodynamic therapy. Sonodynamic therapy (SDT) is loosely defined as the acoustic activation of drug agents, termed sonosensitizers, by coadministered low frequency ultrasound. This sonosensitizer acoustic activation yields localized cytotoxicity, often attributed to the generation of reactive oxygen species (ROS). First explored in the 1990s,12 SDT originated as a proxy for photodynamic therapy (PDT), a minimally invasive treatment paradigm in which light is used to excite photoactive agents (photosensitizers) to a triplet state that subsequently interacts with oxygen and biomolecules to generate ROS.13 Both SDT and PDT thus bear similar abilities to address unresectable tumors, the invasiveness of surgery, and the off-target effects of ionizing radiation and chemotherapy by delivering dualReceived: January 15, 2020 Revised: March 4, 2020 Published: March 4, 2020 A https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review Figure 1. Acoustic cavitation of microbubbles. A driving pressure wave can breach fluid/solid boundaries or imperfections within a fluid, creating a gas pocket during rarefaction that serves as cavitation nuclei. Alternatively, these nuclei can be present as small undissolved gas bubbles in biological media or be administered as preformed exogenous microbubbles/ultrasound contrast agents. During compression, inrushing fluid compresses these endogenous or exogenous microbubbles, causing gas to be dispelled to form a smaller-sized bubble. During rarefaction, gas enters the bubble as fluid is pulled away causing the bubble to grow in size. Together, these cycles of compression and rarefaction create microbubble oscillations, which below a bubble-dependent pressure and size threshold, are termed stable cavitation. If an acoustic pressure beyond the threshold is delivered, the microbubbles will reach a critical upper size limit beyond which they are too unstable to sustain their structure as fluid rushes inward against their growing shell. This causes the microbubble to collapse during the subsequent compression phase, leading to microjetting and bubble implosion. This implosion releases substantial energy, including hot spots with temperatures 4000−25,000 K, the release of light (sonoluminescence) and pressure shock waves >800 atm.17,23−28 enhance sonosensitizer delivery, increase cavitation events, amplify ROS generation, and mediate synergistic combination therapy. targeted, minimally invasive, nonionizing cancer therapy sparing off-target tissue through the use of otherwise benign sensitizers. However, as PDT is inherently hindered by the relatively shallow (<1 cm) penetration depth of light through tissue, its effectiveness against deep-seated tumors in a minimally invasive manner is limited. Capitalizing on the deep tissue penetration of ultrasound, SDT has therefore emerged as a promising alternative to PDT14 for the treatment of deep-seated tumors currently beyond the purview of PDT, while still retaining its advantages over traditional cancer therapies. On the basis of these putative therapeutic advantages, the investigation of SDT has grown in recent years, with 46% of research articles within the field being published in the last 5 years alone (note: obtained by searching for “sonodynamic therapy” within keywords, abstract, or title in Scopus). To date, although preclinical in vitro SDT studies have shown promising results across a variety of cancer cell lines as reviewed by Rosenthal et al.,15 few studies have shown significant longitudinal tumor regression in vivo. And so, in spite of their similarities, SDT has yet to gain the preclinical and clinical success enjoyed by PDT.16 This may largely be attributed to a lack of clarity and consensus surrounding the mechanisms that underly SDT. In the face of such ambiguity and dispute regarding mechanisms within the field, the optimization required for SDT to reach its clinical potential as a novel anti-neoplastic treatment is hindered. Motivated by this limitation, we hope to critically overview the currently proposed mechanisms of SDT and strategies that may advance the field. To this end, we particularly focus on SDT strategies that apply nanomedicines, which have recently become the most widely explored sonosensitizers, encompassing 224 of the 300 SDT studies published in the last 5 years (obtained by searching for “sonodynamic therapy” within keywords, abstract, or title in Scopus). We highlight advantages nanomedicines may offer in enhancing the anticancer therapeutic efficacy of SDT, including their potential to 2. MECHANISMS GOVERNING SONODYNAMIC THERAPY SDT is founded in sonochemical and sonomechanical events that are thought to exert cytotoxic effects through the generation of ROS or mechanical stresses, respectively. These events are the result of microbubble acoustic cavitation17−19 and thus in order to overview the mechanisms of SDT, it is first necessary to establish the types of acoustic cavitation through which it may arise. 2.1. Acoustic Cavitation. As shown in Figure 1, acoustic cavitation involves the formation, growth, and collapse of bubbles in response to a pressure pulse. These bubbles, or cavitation nuclei, can be exogenously delivered (this will be discussed in a subsequent section), or as more prominently explored in the SDT field, generated from a driving acoustic pulse within biological fluids. These cavitation nuclei typically are located at the boundary of a fluid and within gas filled crevices,20 such as the cellular cytoplasm. The existence of these nuclei has been extensively researched and confirmed, while the mechanism behind their creation and stabilization continue to be explored.21,22 Nevertheless, the field of SDT predominantly assumes that cavitation leading to sonosensitizer activation arises from the de novo formation of endogenous microbubbles in response to a driving acoustic pressure wave. Cavitation of these exogenous and endogenous bubbles can be classified as either stable or inertial, each of which yields different bioeffects. At a defined frequency, application of a pressure pulse causes diffusion of gas into the bubble during expansion and out of the bubble during contraction.29 During stable cavitation, bubbles expand and contract around the same resting radius in response to the acoustic field phases of compression and rarefaction30 (Figure 1). At increasing B https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review reported in the form of power density output unless otherwise noted. It is our hope that acoustic pressure will be standardly reported in future SDT studies to better contextualize and understand the bioeffects and proposed mechanical ROSindependent and ROS-dependent mechanisms of action underlying SDT. 2.2. ROS-Based Mechanisms. Given its inspiration from PDT, it is unsurprising that the predominant mechanism of action proposed to instigate SDT cytotoxic effects is through the formation of ROS. Similar to PDT, it is proposed that the release of short-lived ROS causes oxidative damage in proteins, lipids, and DNA.39 Several studies have identified ROS generation following SDT in vitro and in vivo using ROS quenchers,40 probes,41 and electron paramagnetic resonance spin trapping42 across different sonosensitizers and ultrasound parameters. In one such study, Umemura et al.18 observed cell damage after applying the gallium-porphyrin analogue ATX-70 as a sonosensitizer and 4.5 W/cm2 ultrasound to sarcoma 180 (S180) cells. Introduction of 10 mM histidine, a singlet oxygen quencher, was found to rescue cells and exude a sonoprotective effect. Addition of mannitol, a ·OH quencher, did not display such sonoprotection. As such, it was suggested that 1O2 is the predominating ROS that causes SDT effects. Similar results were observed by groups utilizing DCPH-P-Na,42 5-ALA,43 HAC,44 PpIX,45 Photofrin II,46 HpD,41 Rose Bengal,47 Erythrosin B,48 Piroxicam,49 and pheophorbide A50 as sonosensitizers. However, ROS generation beyond 1O2 has also been implicated in mediating SDT effects. For example, the thermolysis of H2O to ·H and ·OH following inertial cavitation-localized-heat generation has also been proposed to be the source of SDT-triggered ROS generation that can either kill nearby cells directly as primary free radicals or undergo free radical oxidative transformation, the products of which are sonosensitizer-dependent.51 Overall, several studies over the last three decades have observed a variety of ROS being generated in vitro following concurrent exposure of cells and solutions to low frequency ultrasound and sonosensitizer52−57 (Figure 2a). Regardless of the identity of ROS generated, the exploration of downstream biological effects has reinforced the school of thought that ROS-based mechanisms predominate SDT phenomena. Such studies looking at ROS-mediated effects have thus far implicated ROS to trigger loss in cell membrane integrity, activation of the mitochondrial-apoptosis pathway58 and oxidative DNA damage,59 ultimately causing cell death (Figure 2b,c). For example, Tang et al. investigated membrane fluidity, morphology, and enzyme activity in vitro in S180 cells posthematoporphyrin SDT treatment with 1.75 MHz, 1.4 W/cm2 continuous wave ultrasound. The authors observed increases in lipid peroxidation and phosphatide degradation with a corresponding decrease in membrane fluidity post-SDT when compared to ultrasound alone, suggesting alterations in membrane structure and lipid composition to play important roles in mediating SDT effects.61 In further studies, cell membrane damage was visually confirmed via SEM images of HL-60 cells exposed to 255 kHz 0.4 W/cm2 ultrasound with or without the administration of the sensitizer merocyanine 540. Clear surface pores and extrusion of the cytoplasm were identified in cells treated with SDT (Figure 2b(i-ii)) but not ultrasound alone (Figure 2b(iv)), indicating cell membrane porosity as a contributor to therapeutic effects.60 These morphological differences between ultrasound alone versus acoustic pressures, nonlinear bubble oscillations arise, creating strong shear forces and viscous shear rates near the surface of the bubble. This nonlinear stable cavitation generates mechanical shearing and microstreaming in nearby environments,31 shown to produce stresses sufficient to disrupt cell membranes.32 Depending on the degree of energy input, this may in turn result in cell death through exposure of the protoplasm to the cell’s external environment. On the basis of their bioeffects, it is these nonlinear oscillations that will henceforth be referred to as stable cavitation within this review. With increasing ultrasound intensities above a given pressure threshold, bubble expansion occurs beyond a given resonant size, leading to a loss of stability. This yields inertial cavitation, whereby inward rushing of fluid against the unstable, expanding bubble causes microjetting, bubble collapse, and implosion (Figure 1). This results in energy release in the form of a shockwave yielding transient (350 ps to <2 μs for a single bubble33) sonochemical hotspots corresponding to a liquid layer roughly 500 molecules thick, modeled temperatures ranging 4000−25,000 K23−25 and pressures above 800 atm.17,26,27 This release of thermal energy may trigger sonochemical reactions generating ROS and sonoluminescence, while the resultant shock waves are capable of mechanically disrupting nearby cell membranes.32,34 As such, while it is anticipated that any SDT therapeutic effects generated in the realm of stable cavitation may be a result of mechanical forces, bioeffects from inertial cavitation may be both sonochemical and sonomechanical in nature. It is thus important to differentiate SDT from the predominantly mechanical effects of thermal and nonthermal HIFU. HIFU thermal ablation makes use of high ultrasound intensities to locally elevate tissue temperatures and cause tissue necrosis.3 Conversely, SDT effects have either shown to be, or are assumed to be, nonthermal or minimally hyperthermic in nature.35,36 Nonthermal HIFU ablation in the form of histotripsy arises from inertial cavitation, but makes use of shorter ultrasound pulses (on the order of μs) and higher ultrasound intensities (exceeding 10 MPa and 1000 W/cm2 for histotripsy versus <10 MPa and <10 W/cm2 for SDT when reported) than typically used in SDT, leading to mechanical nonthermal homogenization of tissue targets.4,37,38 Ultimately, SDT requires the use of a sonosensitizer to impart therapeutic effects that are not solely sonomechanical in nature, making it fundamentally different from HIFU thermal ablation or histotripsy, which does not require any sonosensitizer administration. Consequently, in order to differentiate between stable/ inertial cavitation and between HIFU/SDT effects, reporting of acoustic pressure, beam characteristics, target tissue histology, and detection of cavitation are required. Unfortunately, as will be more thoroughly discussed in later sections, there is a lack of universal reporting of the above parameters within the field of SDT. Field parameters are highly dependent on the ultrasound setup. Given the diverse range of ultrasound apparatus, this absence of apparatus calibration and pressure reporting generates uncertainty when drawing comparisons between SDT studies or when drawing generalized conclusions from any observed biological effects. While we do attempt to differentiate SDT mechanisms and bioeffects based on cavitation phenomena that are pressure dependent, most studies within the field opt to present acoustic intensity in the form of device-dependent power density rather than calibrated pressure measurements. As such, ultrasound parameters will be C https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review Figure 2. continued sensitizer or ultrasound-only controls. Evidence of cell membrane damage was observed by Tachibana et al. when comparing SEM images of HL-60 cells exposed to SDT (i,ii; merocyanine +255 kHz 0.4 mW/cm2 ultrasound) versus those of untreated cells (iii) or cells exposed solely to ultrasound (iv).60 The formation of cell membrane pores has been postulated to be a result of lipid peroxidation from ROS generated post SDT. ROS generation has also been associated with the induction of apoptotic cell death through mitochondrial membrane potential disruption, activation of the Fas death receptor pathway, or changes to the structure of DNA as summarized in (c). Scale bar = 5 μm. The graphic in part (a) was adapted from Cheng et al.54 (Copyright Dove Medical Press Ltd.) and part (b) with permission from Tachibana et al.60 Copyright 1999 Elsevier. SDT combined with the higher degree of lipid peroxidation observed post-SDT in the literature suggest that SDT results in cell membrane damage beyond that of cavitation-mediated effects alone. These observed differences are consistent with previously known ROS-based lipid peroxidation mechanisms, including the ability of 1O2 to react with polyunsaturated fatty acids in the membrane to cause peroxidation of membrane lipids through the lipid hydroperoxide chain reaction.62,63 Therapeutically, this may yield apoptotic pathways similar to other membrane destabilizing cytotoxic agents64 such as potential inactivation of ion channels,65 transport proteins,66 or activation of the Fas death receptor pathway. In addition to loss of cell membrane integrity, real-time loss of mitochondrial membrane potential (MMP) and activation of p53 tumor suppressive pathways have also been associated with SDT. Honda et al. observed a monotonic increase in MMP with increasing ultrasound intensity applied to U937 myelomonocytic lymphoma cells.67 Disruption of MMP has been further implicated in ultrasound-induced apoptosis owing to well-known capabilities of the mitochondria to induce the self-destruct mechanism through caspase activation. Particularly, the disruption of electrochemical gradients observed may be extrapolated to known mechanisms of cristae organization disruption and inhibition of mitochondrial fusion to inhibit function and therefore compromise cellular energy supply.68 Follow-up studies investigating downstream events in conjunction with ROS generation revealed implication of nuclear factor-ΚB and tumor suppressor gene p53 in cellular apoptosis following SDT. Specifically, selective transactivation of p53 target genes was observed following Hp-mediated SDT.69 Later experiments further confirmed activation of the p53/caspase-3 apoptosis axis following sinoporphyrin sodium (DVDMS)-mediated SDT.70 Hyper-physiological levels of p53 are known to influence cellular redox by activating proapoptotic factors PUMA, Bax, and Fas through transcriptiondependent and independent pathways.69 p53 is central in mediating and integrating cellular response to DNA damage, growth factor withdrawal, and oncogenic transformation,71 although these results do appear to be dependent on cell type in SDT. Regardless, ROS-mediated effects on p53 creates intriguing possibilities for combination therapy with SDT given the role of p53 modulation in enhancing combinatorial therapeutic efficacy with chemotherapeutics and radiotherapy.72 This highlights the importance of characterizing the biological effects of SDT, as knowledge of specific downstream pathways may allow for rational sonosensitizer and combination therapy design. Figure 2. Biological effects of SDT are thought to be predominantly caused by ROS generation (a) followed by a loss in cell membrane integrity (b). Cheng et al.54 demonstrated ROS generation in THP-1 macrophages following the administration of 5-ALA-mediated SDT (1 MHz, 10% duty cycle, 100 Hz pulse repetition frequency, 0.5 W/ cm2) using DCFH-DA, an intracellular fluorescent ROS probe. Quantification of fluorescence (n = 6) demonstrated significant intracellular increases in ROS generation following SDT compared to D https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review Figure 3. ROS-dependent SDT mechanisms initiated by inertial cavitation. The resulting sonoluminescence and heat (4000−25,000 K) activate nearby sonosensitizers (shown in green) through (a) a PDT-like mechanism or (b) pyrolysis. Sonoluminescent light is thought to excite photoactive sonosensitizers from the ground state to a short-lived excited singlet state (a−i). Following intersystem crossing, the resulting triplet state can interact with cell substrates and oxygen to generate free radicals through a Type I PDT reaction or can directly interact with molecular oxygen to generate cytotoxic singlet oxygen (1O2) via a Type II PDT reaction. This is made feasible through the emission of sonoluminescent light over a broad range encompassing the Soret band of many porphyrin sonosensitizers as demonstrated by Umemura et al. (a-ii,iii).76 When sonicating solutions (1.93 MHz, 0.6 MPa) containing hematoporphyrin (Hp), a decrease in the intensity of this sonoluminescent light was observed at the Soret band of Hp without any observation of corresponding porphyrin fluorescence at wavelengths >600 nm, suggesting absorption of light by the Hp (a-ii). When Hp and 1.92 MHz, 1.8 W/cm2 ultrasound were administered to Sarcoma-180 cells, decreases in viability were observed that were only discernibly recovered with the addition of the 1O2 scavenger histidine, but not the OH· scavenger mannitol, supporting the proposed involvement of sonoluminescence-mediated Type II sensitizer activation (a-iii). Alternatively, pyrolysis of sonosensitizers may be the source of ROS generation following SDT. The high focal temperatures generated from inertial cavitation can cause the thermolysis of water, yielding free radicals that can interact with sonosensitizers to generate longer-lived cytotoxic peroxyl radicals. Sonosensitizers may also be directly decomposed into radical species in the vicinity of the sonochemical hotspot. The formation of these sensitizer-derived peroxyl radicals was observed by Mišiḱ et al.,77 who observed increasing peroxyl radical generation with increasing sensitizer concentration in solution (ii) that correlated with a decreasing concentration of ·OH. Adapted with permission from Umemura et al.78 (Copyright 1990 John Wiley and Sons) and Mišiḱ et al.77 (Copyright 1996 Elsevier). sonosensitizer activation and greater ultrasound/sonosensitizer synergy in the absence of confounding effects from inertial cavitation. Once generated, it is stipulated that sonoluminescence may stimulate a photochemical reaction similar to that of PDT. Here, the sonoluminescent light may excite sonosensitizers to a short-lived singlet state Sn (Figure 3a(i)). Following intersystem crossing to a triplet state, the sonosensitizer may then interact with biological substrates to generate ROS similarly to a Type I PDT pathway, or by directly interacting with molecular oxygen to generate cytotoxic singlet oxygen (1O2) analogous to Type II PDT. Due to its resemblance to PDT, the sonoluminescent pathway of SDT ROS generation has directed the use of photosensitizers as sonosensitizers. To this end, porphyrins have most widely been explored as sonosensitizers given their ability to undergo photochemical processes to generate ROS via type I or type II PDT.79 Early work from Umemura et al. thus employed porphyrin sonosensitizers to shed light on both the origin and the utility of sonoluminescence in SDT. In initial studies using hematoporphyrin (Hp) as a sonosensitizer,76 the authors observed the emission of visible light between 400 and 450 nm when applying 600 kPa, 1.93 MHz ultrasound to a solution of saline (Figure 3a(ii)). This broad emission was also observed Should a ROS-based mechanism be implicated in SDT, the natural course of action would be to maximize ROS yields in future sonosensitizer design. In order to do so, it is first important to understand the pathways by which sonosensitizers in the vicinity of cavitating bubbles mediate ROS generation. Currently, there are two primary proposed cavitation phenomena that are thought to activate ROS generation: sonoluminescence and pyrolysis (Figure 3). 2.2.1. Sonoluminescence. Sonoluminescence, first observed in 1934 by Frenzel et al.,28 describes the phenomenon through which the energy released from the rapid collapse of a bubble during acoustic cavitation instigates a very brief emission of light. While generally associated with inertial cavitation due to the rapid release of energy resulting from bubble implosion, two studies have reportedly observed sonoluminescence at acoustic pressures and amplitudes attributed to stable cavitation.73,74 Given the lack of extreme energy output in stable cavitation, theoretical models have suggested that the gaseous phase within the bubble can attain Tmax similar to inertial cavitation 75 to generate photons and thereby sonoluminescence via stable cavitation.73 Although minimal study has gone into analyzing the possibilities of such an event, the generation of sonoluminescence under conditions of stable cavitation would promisingly allow for more control over E https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review would combine such sonoluminescence detection techniques (including the in vitro use of photomultiplier tubes, charged couple device surface imaging, or focal ultrasound tomography) with cavitation detection and pharmacokinetically similar photoinactive and photoactive sonosensitizers when exploring ROS generation and associated therapeutic effects from SDT. Furthermore, sonoluminescence detection within mammalian tissue, which remains to be a challenge, may be an avenue that could allow clearer mechanistic conclusions to be derived in vivo. 2.2.2. Pyrolysis. In lieu of sonoluminescence, an opposing theory for ROS-mediated SDT has arisen that postulates sonochemical pyrolysis following inertial cavitation to cause SDT effects. Under this mechanism, cavitation nuclei act as a combustion-chemistry reactor after the extreme energy release from the collapsing cavity. Accordingly, the high local temperatures and pressures generated from inertial cavitation may directly lead to pyrolysis of the bubble components or of the solvent vapor.86 Assuming an adiabatic model of collapse of a spherical bubble, the heat conductivity from the final temperature at the moment of collapse (4000−25,000 K)23−25,34 into the liquid may be sufficient to cause pyrolysis of molecules at the gas−liquid interface to generate free radicals.87 This proposition was supported by Riesz et al.34 who detected the generation of ·OH and ·H following the sonication of aqueous solutions at 1 MHz and 2 W/cm2 intensity ultrasound. Assuming that recombination or disproportionation does not occur, these generated free radicals could act indiscriminately against macromolecules to induce cytotoxic damage. While many studies investigating induction of SDT cytotoxicity through pyrolysis are conducted independent of a sonosensitizer, it is hypothesized that the activation of a drug in proximity to the collapsing sonochemical reaction can amplify the effects generated by ultrasound alone. With the presence of a sonosensitizer, either a lowered inertial cavitation pressure threshold or increased generation of ROS are thought to cause the synergistic effects observed within SDT. However, there remains doubt regarding the contribution of intracellularly generated ·OH following inertial cavitation. Studies have theorized immediate cell destruction in response to intracellular cavitation caused by an oscillating bubble of a size far beyond that of a typical cell.88,89 In this event, the short half-life (∼1 μs for 1O2, ∼1 ns for ·OH),90 high reactivity, and limited diffusion distances of ROS (∼20 nm for 1O2, ∼5 nm for ·OH)91 may limit their involvement in imparting cytotoxicity following inertial cavitation. Assuming extracellular cavitation, there may exist limitations by which ROS can react with critical cellular sites such that only those proximal to the area of production are affected.92 Based on this, it has been proposed that pyrolysis of sonosensitizers may create free radical intermediates beyond ·OH and H·, such as peroxyl radicals. These less reactive, longer-living radicals may then, in theory, be able to migrate the necessary distances to cell membranes where lipid peroxidation and cell death would occur.93 Various studies contradicting this postulate typically revolve around the protective effect of cysteamine, a cell membrane-permeable free radical scavenger, and the lack of protection provided by cystamine, a membrane-impermeable free radical scavenger94,95 against SDT. Further information contending this debate can be found in extensive detail in reviews by Church et al.96,97 and Miller et al.88 with the addition of ROS scavengers, specifically those acting on 1O2, OH·, and O2−, suggesting that sonoluminescence was a result of cavitation hot spots rather than from the recombination of ROS. Noting the overlap between the peak absorbance of Hp at 411 nm80 and the broad sonoluminescence peak between 400 and 450 nm, the authors hypothesized activation of the sonosensitizer either through nonequilibrium energy transfer from sonochemical hotspots or by direct photoactivation from the generated light. On the basis of this study, multiple papers have investigated sonoluminescence as a mechanism of SDT action in vitro and in vivo.41,81,82 Umemura et al.’s seminal work also illustrates the requirements needed for a PDT-like sonoluminescence pathway of SDT action to ensue: (1) a sensitizer with a high yield of intersystem crossing to generate an excited triplet state, and (2) overlap between the peak absorbance of the sensitizer and the broad emission of sonoluminescence. It should also be noted that only through the triplet state can energy be transferred to nearby O2 molecules to generate 1O2. Thus, the observation of 1O2 generation in SDT studies is often associated with the presumption that sonoluminescence is the source of sensitizer activation. There have, however, been studies that contradict sonoluminescence-mediated photodynamic generation of ROS as the SDT mechanism of action. Of particular interest was a study that made use of 13,17-bis(1-carboxyethyl)-8-[2(2,4-dichlorophenyl-hydrazono)ethylidene]-3-ethenyl-7-hydroxy-2,7,12,18-tetramethylchlorin, disodium salt (DPCH-PNa), an agent claimed to be photoinactive.83 Despite presumed photoinactivity, Hachimine et al. observed statistically significant sonotoxicity in vitro and in vivo when compared to ultrasound alone at 1 MHz, 2 W/cm2, contradicting the belief that SDT activity is based on sonoluminescent photoactivation of sensitizers. Sonotoxicity on cell viability was blocked and reached ultrasound-alone levels with the addition of histidine but not mannitol, suggesting the involvement of 1O2. However, although claimed to be photoinactive, DPCH-P-Na did cause statistically significant cell toxicity when compared to controls upon photoirradiation with 60,000 lx for 10 min, making it difficult to exclude sonoluminescence completely from a mechanistic perspective. Nevertheless, other studies have also demonstrated the generation of ROS from sensitizers that would typically be photoinactive. For example, copper protoporphyrin (Cu-PP) was found to produce cytotoxic effects in response to albumin microbubble-mediated SDT using 3.2−4.0 W/cm2 acoustic intensity spatial peak temporal average on L1210 cells.84 This result suggested that sonoluminescence was not responsible for the cytotoxic effects observed, as the short triplet state lifetime of the metalloporphyrin would yield poor generation of 1O2 upon photoexcitation. Other compounds, such as piroxicam, have also been explored to induce cytotoxic effects from 1O2 generation49 despite their low singlet oxygen yield.85 Collectively, these studies suggest that SDT is governed by mechanisms of action beyond simple sonoluminescencemediated PDT-like photoactivation of sonosensitizers. Indeed, the exclusive observation of efficacy resulting from porphyrin-derived sonosensitizers is insufficient as conclusive evidence for sonoluminescent SDT activation. Further comprehensive studies with clinically relevant ultrasound parameters, light controls, sonoluminescent detection techniques, and ROS probes with high specificity are required to investigate this possibility. Ideally, these mechanistic studies F https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc To this end, water-based azo compounds have been explored as sonosensitizers due to their ability to decompose to carbon-centered alkoxyl radicals and ultimately peroxyl radicals in the presence of oxygen.52 Both radical species were successfully identified after sonication through spin trapping and electron paramagnetic resonance spectroscopy, indicating successful decomposition of the sonosensitizer. Indeed, quenching with potassium iodide, sodium azide, and sodium formate did not decrease the concentration of radicals present, suggesting a thermolysis/pyrolysis-mediated effect of the sonosensitizer as opposed to an 1O2 or ·OH-mediated response. Crucially, however, these experiments were conducted in vitro at low frequency ultrasound (50 kHz) in a standing wave field, whereupon efficacy is often directly related to a given spatial average, temporal average ultrasound intensity.98 Variations in geometry between ultrasound systems make it difficult to compare cavitation or estimate acoustic intensity delivered. Standing wave production is also known to promote cavitation by virtue of bubbles below resonant size collecting at pressure maximums.99 Collectively, this nonstandard ultrasound delivery prevents the observation of peroxyl-mediated cytotoxicity observed in this study to be extended broadly, necessitating further studies to explore the mechanism of decomposition of azo compounds using translatable ultrasound parameters. As a whole, pyrolysis within SDT is not well understood and the products produced from different classes of sonosensitizers remain to be elucidated. Mechanistic studies that make use of sonosensitizers that can amplify pyrolysis may thus be of interest. 2.3. ROS-Independent Mechanisms. While ROS-mediated effects are generally accepted as the dominant mechanism underlying SDT, one must account for the inherent sonomechanical effects induced by ultrasound. Ultrasound irradiation alone can hydrodynamically shear cells through acoustic microstreaming, pressure pulse-mediated erosion, and microjetting, all of which can ultimately cause cell membrane disruption.100−102 Initial work conducted by Worthington et al.103,104 supported sonomechanical events leading to SDT effects as opposed to ROS generation. The authors investigated HO· and H· yield through Fricke dosimetry following 1.955 MHz variable intensity ultrasound (where 1.2 W/cm2 ≈ 0.19 MPa) applied to both water and PBS, with or without hematoporphyrin (Hp), and shaken or tilted to stimulate microbubble formation. No singlet oxygen luminescence could be measured with or without Hp post-36 W/cm2 ultrasound despite confirmation of cavitation signal, notable cell death, and detectable luminescence using corresponding PDT treatment. Given the intensities used, it comes as little surprise that sonomechanical damage was interpreted to be the predominant mechanism of cell death given the known associated cavitation and potential thermal effects. While this study fails to incorporate any clinically relevant ultrasound parameters in its SDT studies, it may loosely be taken to provide opposition toward a ROS-based theory that has yet to be fully addressed. Although the mechanical effects of acoustic cavitation alone have been widely studied, it remains unclear as to how, or if, the addition of a sonosensitizer will mediate a synergistic cytolytic response. Current theories propose that the addition of a membrane destabilizing compound can enhance physical stresses induced by ultrasound to mediate a synergistic effect (Figure 4). While few studies have looked in depth at sonomechanical effects in the presence of a sensitizer, previous Review Figure 4. ROS-independent mechanisms of SDT actions. It is thought that sonosensitizer incorporation within cell membranes causes their destabilization. This in turn is thought to make the cell membrane more vulnerable to cavitation mechanical events such as microjetting, microstreaming, and shock waves resulting from inertial cavitation. Thus, sonosensitizers may enact their synergistic SDT effects by lowering the pressure threshold needed for sonomechanical cell damage and subsequent death. research analyzing acute mechanically induced hemolysis suggests a role of drugs, in this case, Vitamin E, in making cells more vulnerable to lysis.105 Follow-up studies employing Trolox, a derivative of Vitamin E, demonstrated that the drug enhanced shear forces following ultrasound-induced hemolysis.106 This enhanced membrane-destabilization via drug agents may in turn be applied to SDT. It will therefore be of interest to characterize the interactions between sonosensitizers and cell membranes to elucidate how, or if, this interaction amplifies cavitational effects. Porphyrins in particular have previously been modeled to interact with cell membranes through several types of forces: electrostatic, hydrophobic, hydration, and coordination. The hydrophobic porphyrin core is known to embed into the lipid region of cell membrane bilayers, creating not only preferential accumulation of the molecule, but also potential enhancement of mechanical stresses by destabilizing the membrane.107 It is important to note that while these theorized porphyrin−membrane interactions may lead to enhanced sonomechanical effects, they are thus far based on photosensitizer-membrane modeling performed for PDT and not SDT. Given the well-known role of sonomechanical damage in ultrasound, it is worth exploring its impact within SDT across the different sonosensitizers in use. Furthermore, exploration of such presumed sonomechanical events within the realms of both stable and inertial cavitation may lead to better clarification of the contribution of mechanical versus ROS-mediated effects. For example, when applying stable cavitation from ultrasound alone to U937 cells, G https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review Table 1. Summary of Proposed Mechanisms Underlying the Effects of SDT Mechanism Supporting Evidence Contradicting Evidence Unknowns Sonoluminescence Overlap observed between peak absorbance of porphyrin sonosensitizers and sonoluminescence peak Triplet state to generate 1O2 − observed through electron paramagnetic resonance spin trapping, probes, quenchers ·OH, H·, long-lived peroxyl and alkoxyl species detected via electron paramagnetic resonance spin trapping, probes, quenchers, indicating decomposition of sonosensitizer Short half-life of ROS limits migration within cellular sitesmust require longer-lived radicals to mediate observed therapeutic response Known ability of ultrasound to cause physical damage Membrane destabilizing compounds enhance ultrasound-induced hemolysis Metalloporphyrin and other compounds with low singlet oxygen yield (ex, piroxicam) yield cytotoxic and therapeutic efficacy despite short triplet state lifetime Intensity, power, duration, wavelength of light emitted from sonoluminescence Characterization of light emitted in relation to the class of and structure of sonosensitizer used Products produced from different classes of sonosensitizers can vary greatly in both structure as well as mechanism of pyrolysis 41,76,81,82 Impossible to isolate physical stressors from the generation of ROS during inertial cavitation 88,101,102,105,106,108 Pyrolysis Mechanical Protective effect observed by cysteamine, a cell membrane-permeable free radical scavenger, and lack thereof by cystamine, a membraneimpermeable scavenger, suggesting activity of ROS within the cellular membrane Generation of ROS in most studies likely indicates this is not the sole mechanism at play Refs 34,52,88,91,94−97 comprehensively explore the contribution of sonoluminescence, pyrolysis, and mechanical effects in vitro and in vivo across different classes of sonosensitizers and cavitation paradigms (stable and inertial) is warranted. This will allow for better elucidation of sensitizer-specific mechanisms of SDT and the derivation of structure−activity relationships for optimal sonosensitizer development and ultrasound parameter selection. Feril et al. showed neither free radical detection nor oxidative stress response despite inducing 70.0 ± 13.8% apoptosis in vitro.108 Increasing the applied acoustic pressure was associated with both free radical detection and oxidative stress response, as well as further decreases in cell viability and increases in secondary necrosis, showcasing the combinatorial sonomechanical and sonochemical effect known to be a part of inertial cavitation. Since it is impossible to isolate physical stressors from the generation of ROS during inertial cavitation, studies focusing on SDT under parameters associated with stable cavitation may shed light on the mechanical contributions of acoustic cavitation in SDT. 2.4. Summary of Mechanisms and Future Outlook. The driving force behind SDT is the interaction of sonosensitizers with cavitating bubbles in biological media through ROS-dependent and independent mechanisms, as summarized in Table 1. Acoustic cavitation, specifically inertial cavitation, can generate hot spots giving rise to high temperatures and sonoluminescence that can in turn trigger the generation of sonosensitizer-derived ROS, although stable cavitation could also be implicated in ROS generation. Sonosensitization may alternatively or additionally be a result of drug-induced membrane destabilization, lowering the threshold for cell membrane damage by sheer forces generated from acoustic cavitation. However, studies thus far exploring the therapeutic utility and potential mechanisms at play in SDT largely indicate that therapeutic effects, particularly in vitro, are mediated through ROS. This overarching basis of SDT was also supported in vivo by McEwean et al., who demonstrated that oxygen-containing microbubbles suppressed tumor growth to a greater extent than equivalent microbubbles loaded with sulfahexafluoride gas following Rose Bengalmediated SDT in BxPC3 subcutaneous tumor-bearing mice.109 The observation of oxygen-amplified therapeutic effects supports a ROS-mediated therapeutic mechanism of action, which would require the presence of molecular oxygen. Nevertheless, the overarching pathways and patterns leading to such ROS generation remain unclear. Given the differences in physiochemical properties across sonosensitizers, it is logical to assume that each class follows different means of acoustic activation with differing degrees of contributions from each of the mechanisms proposed. Thus, mechanistic studies that 3. SONOSENSITIZERS Thus far, we have briefly overviewed how SDT is instigated in the context of acoustic cavitation. Understanding such mechanisms may allow for more purposeful sensitizer selection and thereby improved therapeutic outcomes. Advances in sonosensitizer chemistry in parallel are also imperative for congruently establishing greater SDT efficacy. Traditionally, SDT has made use of molecular sonosensitizers. Multiple comprehensive literature reviews have detailed the diverse range of molecular sonosensitizers used preclinically110−113 and thus will not be covered in depth within this review. Thus far, numerous classes of molecular sonosensitizers have been used in literature including porphyrins,76 xanthene dyes,47 chemotherapeutic anthracycline derivatives,114 azo-compounds,52 heptamethine dyes,115 nonsteroidal anti-inflammatory drugs (NSAIDs),49 and many more. However, it is debatable whether the observed increases in cytotoxicity in some of these drug classes were due to sonodynamic activation of these drugs as would be expected for SDT, or merely a result of increased drug delivery. Accordingly, sonosensitizers without inherent toxicity have been primarily researched to better differentiate SDT synergistic effects from those imparted by the drug itself. Particularly, porphyrin-based photosensitizers are of great focus owing to their propensity to produce ROS upon photoactivation. Their ubiquitous use, however, places a greater emphasis on the role of sonoluminescence in SDT than would be with the use of photoinactive sonosensitizers. As such, there remain many avenues for sonosensitizer innovation that may enhance SDT efficacy and translation. To this end, supramolecular sonosensitizer assemblies, particularly in the form nanoparticles, have recently advanced the therapeutic efficacy and feasibility of SDT as a cancer treatment strategy as will be discussed herein. H https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review Figure 5. Enhancing SDT through nanomedicines. Encapsulation of sonosensitizers within nanoparticles can improve bioavailability and increase drug delivery to cancer cells by increasing drug solubility, facilitating accumulation via the enhanced permeability and retention effect, cell-specific targeting and on-demand ultrasound-triggered sensitizer release (a). This was exemplified by Zhang et al.129 who demonstrated that encapsulation of HMME within liposomes allowed for ultrasound-mediated drug release (i), higher tumor accumulation of liposomal ICG versus free ICG (ii), and ultimately delayed tumor growth of liposomal HMME following the administration of SDT to MCF-7 tumor-bearing mice (iii) (adapted with permission from ref 129, Copyright 2019 John Wiley and Sons). Nanoparticle formulation of sonosensitizers also holds the advantage of providing alternative pathways to sonosensitization (b). This can include the use of inorganic nanoparticles, such as (i) TiO2 as a new class of ROSgenerating sonosensitizers (adapted with permission from Harada et al.,131 Copyright 2013 Royal Society of Chemistry), (ii) increasing microbubble cavitation in solutions (adapted with permission from Tuziuti et al.,142 Copyright 2005 American Chemical Society), and (iii) creating more cavitation nuclei in solutions containing nanoparticles (bottom two panels), versus solutions devoid of nanoparticles (top two panels; adapted with permission from Pan et al.,143 Copyright 2018 John Wiley and Sons) to enable improved therapeutic efficacy. ROS generation can also be amplified by nanomedicines through the codelivery of sonosensitizers with oxygen to overcome tumor hypoxia, coloading with agents activated by SDT-induced hypoxia to generate ROS and reducing intratumoral GSH levels to prevent scavenging of ROS generated by SDT (c). Coloading of oxygen with the sonosensitizer IR780 increased ROS generation intracellularly (i), which translated into increased survival compared to SDT conducted in the absence of oxygen loading (ii) (adapted with permission from Chen et al.,144 Copyright 2017 American Chemical Society). Conversely, SDT itself can result in consumption of oxygen and tumor hypoxia (iii) as evidenced by observable HIF-1α staining of PC3 tumor slices following the application of SDT in comparison to control tumors.145 This tumor hypoxia was exploited to activate the hypoxic prodrug TMZ I https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review Figure 5. continued to enable bioreductive therapy (BRT) that delayed tumor growth compared to SDT alone (iv) (adapted with permission from Wang et al.145 Copyright 2018 John Wiley and Sons). Finally, coencapsulation of multiple therapies within a single nanomedicine agent lends itself to spatially colocalized combination treatments and synergistic therapy, including chemo/SDT, PDT/SDT, radiotherapy/SDT, immune/SDT, which can overcome drug resistance and prevent tumor metastasis (d). The immunomodulatory abilities of SDT combined with TLR7 agonist therapy from a single nanoparticle agent was demonstrated by Yue et al.146 The combination therapy increased dendritic cell maturation (i) and the release of cytokines such as TNF-α (ii), which, when combined with anti-PD-L1 therapy, decreased primary and distant tumor growth (iii-iv) (adapted with permission from ref 146, Copyright 2019 Springer Nature). 3.1. Role of Ultrasound Contrast Agents. Exogenously delivered microbubbles have served as supramolecular diagnostic agents for ultrasound imaging for decades. Approved clinically to visualize tissue and cavity perfusion, ultrasound contrast agents are micron-sized (1−8 μm diameter) gas-filled microspheres surrounded by a lipid, polymer, or protein shell in colloidal suspensions.116 As their acoustic behavior is similar to that of endogenously formed bubbles, it is proposed that microbubbles can lower the threshold for SDT events by acting as preformed cavitation nuclei,117,118 ultimately yielding higher therapeutic efficacy at equivalent or lower acoustic intensities than agent-free platforms.119 Preformed microbubbles, similar to de novo bubbles, also have the ability to generate sonoluminescence at intensities associated with inertial cavitation.120 Inclusion of sonosensitizers within the microbubble shell can further increase SDT-mediated cytotoxicity in a bubble-dependent manner. For example, in examining the effects of Rose Bengal (RB) either covalently attached to lipid microbubbles or independently administered as a free drug agent alongside lipid microbubbles under ultrasound irradiation (1 MHz frequency and 1.5 W/cm2 power density for 60 min), Nomikou et al. observed that the RB-microbubble conjugate increased cell death in vitro compared to the unconjugated RB + microbubbles and sonosensitizer-alone SDT treatment groups.119 In vivo studies were attempted using more therapeutically relevant ultrasound parameters (1 MHz frequency and 3.5 W/cm2 power density for 3 min) showing no increase in LNCaP-Luc tumor growth with RB-microbubble conjugate SDT after 12 days, although an in vivo comparison to unconjugated RB was not conducted.119 Similar promising results have been observed with microbubble-conjugated chemo-sonodynamic therapy,121,122 as well as oxygen/RB-loaded microbubbles for hypoxic tumor treatment.123 Thus, sonosensitizer-loaded microbubbles may be a promising step forward toward advancing the therapeutic effects of SDT, provided that ultrasound dosimetry is clearly evaluated in a standardized manner (for example, through acoustic pressure, and with comparison against effects observed for free sensitizer and microbubble-devoid control groups). 3.2. Role of Nanomedicine in SDT. While microbubble agents amplify SDT effects by acting on acoustic cavitation, nanoparticles deliver advantages more pertinent to sonosensitizer availability and activity. As such, nanoparticles account for the most widely explored supramolecular variant of sonosensitizers in the literature. More specifically, nanosensitizers can increase SDT efficacy by better facilitating sonosensitizer delivery, generating new means of ROS generation, overcoming hypoxia, and allowing for spatially localized combination therapies to be explored (Figure 5; Table 2). 3.2.1. Drug Delivery. Recent decades have witnessed an abundance of preclinical exploration of nanosized materials to change the pharmacokinetics of free drug agents and maximize their on-target delivery. Numerous studies using liposomal, micellar, and polymeric nanoparticles within the scope of chemotherapy drug delivery have shown cell targeting, delivery across biological barriers, reduction in toxic side effects, and avoidance of multidrug resistance protein 1 (MDRP1).124,125 Similar delivery advantages can be extended to nanoformulations of sonosensitizers. The hydrophobic nature of many traditional organic sonosensitizers, such as porphyrin, can reduce their bioavailability and, accordingly, therapeutic concentration in vivo. Assembling these free sensitizers within nanostructures can potentially overcome this challenge, allowing for higher drug doses to reach tumor sites.126 Furthermore, the dense packing of sonosensitizer within a single nanoparticle could allow for greater therapeutic efficacy given the need for spatiotemporal localization of ultrasound and sensitizers for effective SDT. Consequently, nanoparticle systems may facilitate more potent SDT by improving sonosensitizer delivery to target sites. To this end, hollow mesoporous organosilica nanoparticles (HMONs) have been explored as nanocarriers due to their large surface area and pore volume.127 These traits can respectively allow for dense covalent anchoring of sonosensitizers (PpIX) to maximize drug delivery, and for diffusion of SDT-generated ROS out of the mesopores. When exposed to PpIX-HMON SDT (1.0 MHz, 1.5 W/cm2, 2 min) in vitro, a reduction of more than 70% cell viability was observed in 4T1 cells, while the nanoparticle itself showed no cytotoxicity. When applied in vivo at 2.3 W/cm2, statistically significant tumor growth inhibition was achieved over ultrasound alone. In another drug loading study conducted by Liu et al.,128 sinoporphyrin sodium (DVDMS) was loaded into homotypic tumor cell-derived exosomes. The authors observed superior tumor suppression and ROS generation compared to both free DVDMS as well as nonirradiated exosomes in a reportedly synergistic manner. Delivery was also enhanced when the nanoformulation of DVDMS was combined with ultrasound treatment. Intracellular localization was observed to shift from the lysosome to the mitochondria following US stimulation, suggesting promotion of cargo transport and endosomal opening to trigger cargo release. Enhanced tumor delivery and penetration in vivo were further observed with ultrasound versus without. This phenomenon of cavitation-mediated drug release was further observed by Zhang et al.129 The authors demonstrated that hematoporphyrin monomethyl ether (HMME)-loaded liposomes could rapidly release 60% of the loaded HMME when exposed to 1 MHz, 0.5 W/cm 2 ultrasound (Figure 5a). This translated to higher accumulation of loaded imaging agents in MCF-7 tumors exposed to ultrasound when compared to free agents, and ultimately slightly slower tumor growth with the use of liposomal versus free HMME. Thus, nanoformulations of sensitizers may facilitate increased drug delivery to target lesions, possibly by facilitating triggered drug release. J https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review Table 2. Summary of Nanomedicines Explored for SDT Nanoparticle HMONs Sensitizer PpIX Function/Effect Drug Delivery Protected sonosensitizer from physiological environment, enhanced tumor accumulation, sustained release US Frequency 1 MHz DVDMS Intensity/ Pressure Ref 1.5−3.3 W/cm2 2−3 W Pressures unknown 127 128 Liposomes Pyropheophorbide Triggered delivery of coloaded sonosensitizer 1 MHz 0.2−0.3 W/cm2 Pressures unknown 130 PFP-Nanobubble TPPS derivatives Acted as cavitation nuclei, enhanced sensitizer tumor penetration 3 MHz 1.8 W/cm2 Pressures unknown 158 PFC-Nanodroplet IR780 Enhanced sensitizer tumor penetration via diffusion and ADV vascular disruption 1.2 MHz 650 kHz 4.5−6.0 W 2.4 W/cm2 Pressures unknown 159 160 TiO2 TiO2 Alternate Sonosensitization Metal-based 1O2 generation 1 MHz 0.1−1.2 W/cm2 30 W 1.0 W/cm2 Pressures unknown 131,133−135,137,138 1.5 MHz Not listed 132,139 140 AuNPs Au Metal-based ROS generation (plasmonic effect) 1.7 MHz 0.008−0.080 mJ/cm2 Pressures unknown 148 NiFe2O4/C Graphene Promotion of ROS production, potential hyperthermia application 1 MHz 1.0 W/cm2 Pressures unknown 149 Metal−Organic frameworks PMCS Metal-based ROS generation (gap in between occupied/ unoccupied molecular orbital) 1 MHz 2.5 W/cm2 Pressures unknown 143 Silica NPs None Cavitation nuclei, enhancement of hyperthermia 0.88/2.64 MHz Not listed 1.0−2.0 W/cm2 2W Pressures unknown 151 Rose Bengal 150 PTFE NPs N/A Lower cavitation threshold, higher hydroxyl yields 2 MHz 2.0−5.0 MPa 161 HMON + O2 HMME IR780 Amplifying ROS Generation Oxygen delivery to hypoxic tumors to improve ROS generation 3 MHz 1 MHz 5 W/cm2 1 W/cm2 156 144 HMON + TPZ Chlorin e6 Hypoxia-triggered ROS generation via TPZ 1 MHz 1 W/cm2 145 HMON + Ferrate (VI) PpIX GSH depletion for improved local ROS yield 1 MHz 1.4 W/cm2 Pressures unknown 56 PtCu3 PtCu3 Act as horseradish-peroxidase-like enzymes, catalyzing H2O2 into ·HO and as GSH-Px-like enzyme to deplete GSH and increase ROS for chemodynamic enhanced SDT 35 kHz 3.0 W/cm2 Pressures unknown 152 K https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review Table 2. continued Nanoparticle Sensitizer TiO2 + TPZ TiO2 Fe (III) MON + SOD2 siRNA TPPS Liposome + Dox Chlorin e6 US Frequency Function/Effect Amplifying ROS Generation Hypoxia-triggered ROS generation via TPZ Intensity/ Pressure Ref 1 W/cm2 Pressures unknown 157 High ROS efficiency, FL/MR imaging, vehicle to deliver 1 MHz siRNA, reduction of GSH levels, generation of Fenton for chemodynamic enhanced SDT 0.56 W/cm2 Pressures unknown 162 Combination Therapy SDT-triggered Dox release for SDT + chemo 2 W/cm2 163 1 MHz 1 MHz Liposome + HMME immunoadjuvants Co-checkpoint-blockade immunotherapy for antimetastatic 1 MHz response 1.5 W/cm2 146 Liposome Zinc Phthalocyanine PDT + SDT 1.1 MHz 1 W/cm2 (ISATA) Pressures unknown 164 TiO2+ Dox TiO2 ROS-triggered Dox release for SDT + chemo 1.5 MHz 3−15 W/cm2 141 TiO2 Black TiO2−x Enhanced electron−hole separation for enhanced sonocatalytic efficacy to mediate SDT + PTT 1 MHz 1.5 W/cm2 165 Au-TiO2 TiO2 Enhanced ROS yield and NIR II red shift for improved SDT + PTT 3 MHz 0.5 W/cm2 Pressures unknown 166 Polymeric + PTX IR780 SDT-triggered release for SDT + chemo 1 MHz 0.1−0.4 W/cm2 167 Polymeric + Dox Chlorin e6 SDT + chemo with slight immunological response observed 1 MHz 1.0 W/cm2 Pressures unknown 168 MSNs + curcumin TiO2 SDT-triggered curcumin release for SDT + chemo 1 MHz 2 W/cm2 Pressures unknown 169 Dendrimer + Dox Indocyanine Green SDT-triggered Dox release for SDT + chemo 1.20 MHz 1−3W Pressures unknown 170 AuNPs Au Enhanced cell killing observed post-2 Gy X-ray RT + US 1 MHz 0.5−1.5 W/cm2 Pressures unknown 171 Janus Pt-CuS TAPP PTT controls TAPP release and elevates O2 level to modulate SDT therapeutic efficacy 1 MHz 1.0 W/cm2 Pressures unknown 172 The mechanism behind such triggered drug release was touched upon by Wang et al.130 using liposomes loaded with doxorubicin and pyropheophorbide-lipid, termed Dox-pp-lipo. When exposed to 0.3 W/cm2 intensity ultrasound, the authors observed increased doxorubicin release in solution compared to liposomes devoid of pyropheophorbide. The authors suggested that ROS generated from pyropheophorbide sensitization by ultrasound could enable lipid oxidation of the nanoparticle shell and subsequent release of the loaded doxorubicin. This was supported by the observation of ·OH generation in solution following sonication, and reduction in doxorubicin release following the addition of the 1O2 scavenger NaN3 to the liposomal solutions. Improved in solution and intracellular doxorubicin release translated to an increase in median survival of U87-bearing mice by 23 days when administered Dox-pp-lipo + ultrasound versus doxorubicin alone. Based on the sonodynamically dependent on-demand drug delivery elucidated within the above studies, it is of great interest within the scope of SDT to further optimize this novel approach, not only as a means of improving sonosensitizer L https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review have contributed to the cavitation effect observed. Further studies confirming the role of nanoconstructs to act as and stabilize cavitation nuclei have been performed using mesophorous silica nanoparticles,153 single-cavity polymeric nanoparticles (nanocup),154 and polytetrafluoroethylene nanoparticles.155 Should nanoparticles indeed serve as nuclei for acoustic cavitation, they should lower the pressure threshold required to enable SDT effects, similarly to microbubble contrast agents. However, nanoparticles hold the added advantage of being able to extravasate beyond the vasculature. Thus, nanoparticles hold the capacity of more broadly augmenting SDT both intra- and extravascularly, regardless of whether the mechanisms underlying their therapeutic response is attributable to mechanical shearing, pyrolysis, or sonoluminescence. This advantage can be further extended through the use of nanodroplets and nanobubbles as will be discussed below when considering the future outlook of nanomedicines in SDT. 3.2.3. Amplifying ROS Generation. In addition to serving as potential cavitation nuclei, nanoformulations of sonosensitizers may also amplify the generation of ROS by overcoming or exploiting tumor hypoxia. This advantage is centered around the ability of nanoparticles to house multiple drugs within a single agent. Presuming that a ROS-dependent SDT mechanism of action predominates observed therapeutic effects, the codelivery of oxygen with sonosensitizers should increase ROS generation, especially in hypoxic tumor environments. This indeed has been observed for nanoformulations combining sonosensitizers and oxygen-carrying perfluorocarbons.144,156 Coloading of oxygen and IR780 within HMONs proved to increase intracellular ROS production and incited cell death in otherwise SDT-resistant PANC-1 cells.144 Impressively, this combination SDT/oxygen therapy decreased tumor hypoxia compared to control tumors and increased the survival of subcutaneous PANC-1 tumor-bearing mice over a 60-day period compared to SDT conducted without oxygen coloading (Figure 5c,i-ii). It should be noted that this was one of the few studies within the field of SDT that demonstrated such longitudinal therapeutic effects. Alternatively, it is believed that SDT itself can yield a hypoxic tumor environment through the consumption of molecular oxygen during sonochemical reactions between cavitating bubbles and proximal sonosensitizers. Wang et al. took advantage of this phenomenon to amplify ROS generation through the activation of tirapazamine (TPZ), a hypoxia prodrug that is only activated into its free radical form under hypoxia.145 The authors coloaded TPZ, Holmium (imaging agent) and chlorin-e6 (Ce6; sonosensitizer) into hollow mesoporous silica nanospheres modified with APTES and conjugated to mAbPSCA for tumor cell targeting. SDT ROS generation was confirmed only in the presence of Ce6 via quenchers, reaffirming the necessity of the sonosensitizer for ROS generation. Both pH- and GSH-responsive controlled drug release was observed in solution with less than 10% leakage under normal physiological conditions. This translated into cytotoxic effects in vitro, reducing PC-3 cell viability to <20% following nanoparticle and ultrasound treatment. As postulated by the authors, nanoparticle-mediated SDT created a hypoxic tumor environment (Figure 5c,iv) that the authors exploited in vivo to deliver SDT and bioreductive therapy (BRT). The authors observed 85% tumor growth inhibition by day 14 after SDT (particles with Ce6 and no TPZ) and 91% tumor growth inhibition was observed with SDT and BRT delivery, but also more broadly to improve drug release from existing chemotherapy nanoparticle formulations. This advancement in the field of SDT would be analogous to PDTtriggered drug delivery, whereby SDT-triggered release holds the potential of targeting deeper tumor tissues. 3.2.2. New Avenues for Sonosensitization. While current sonosensitizer design philosophies are centered around the application of PDT molecular sensitizers, the use of nanomedicine within the field of SDT has opened the door to delivering sensitization through inorganic nanoparticles. Inspired by multidisciplinary work in catalytic chemistry, titanium oxide nanoparticles (TiO2),131 Au deposition on TiO2 nanoparticles,132 and graphene oxide integration onto TiO2 nanoparticles133 were applied to facilitate electron−hole separation to amplify ROS generation following SDT. A U251 glioma cell line exposed to TiO2 and 1.0 MHz 1.0 W/cm2 ultrasound for 50 s showed equal cell toxicity when compared to 18 J/cm2 UV light irradiation at 5.0 mW/cm2. While photodynamic toxicity was almost completely inhibited by the addition of glutathione, a free radical scavenger, suppression of sonodynamic toxicity was not significantly observed following glutathione addition.134 This suggested that TiO2 may facilitate sonosensitization in a manner untraditionally associated with photoactive sensitizers. Similar toxicity results have also been shown with C32 human melanoma cells135 and HSC-2 human carcinoma cells.136 Owing to the low ROS quantum yield of traditional TiO2 nanoparticles from fast electron−hole recombination, conjugation and functionalization have also been explored using pre-S1/S2 antibody recognizing hepatocytes,137 avidin immobilization,138 hydrophilization with carboxymethyl dextran (CMD),139 and autophagy regulation loading140 to enhance cellular uptake and increase cellular concentrations. More recent studies have investigated combinational sonodynamic chemotherapy using doxorubicin-coordinated TiO2 nanoparticles capable of tumor targeting, controlled drug release, and intracellular ROS generation.141 Although not directed toward SDT specifically, an in-depth overview of the conceptual enhancement of cytotoxicity by TiO2 nanoparticles with exposure to ultrasound has been explored by Shimizu et al.147 Given these initially promising results, further exploration of TiO2 nanoparticle SDTmechanistics may yield more efficacious avenues for sonosensitization and better inform the current understanding of SDT mechanisms of action. Other inorganic nanoparticles explored within literature include gold,148 nickel ferrite/carbon,149 iron oxide,150 silicon,151 platinum,152 and graphene,133 the mechanisms of action of which also need to be better eludicated. One potential means by which inorganic nanoparticles may facilitate or augment sonosensitization is by acting as cavitation nuclei. In 2005, Tuziuti et al. demonstrated that adding alumina particles to an aqueous solution increased the presence of harmonics associated with bubble cavitation, and also increased the temperature of the solution, presumably by increasing the number of sonochemical hotspots generated142 (Figure 5b,ii). This was followed more recently by Pan et al.143 who developed a metal−organic framework-derived carbon nanostructure containing porphyrin-like metal centers (PCMS). Movies captured of the PCMS cavitation effects demonstrated identical growth and collapse of cavitation bubbles, but with an increased number and size of cavitation clusters in PMCS-containing solution when compared to ultrasound irradiation of water alone (Figure 5b,iii). The observation of microjets in US-irradiated PMCS solutions may M https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review combinations have been explored to impede tumor metastasis and exert synergistic therapy. Treatment of 4T1 xenotransplanted mice with DVDMS (sonosensitizer), 2-deoxyglucose (glycolysis suppressor), and 1.90 MHz 2 W/cm2 ultrasound resulted in a slight decrease in pulmonary nodule count when compared to SDT alone. The effect against metastatic tumor tissue was attributed to an observed downregulation of both HK2 and Glut1 expression, leading to reduced levels of glycolysis in tumor tissue. This prevented cellular proliferation and metastases through alterations in cellular homeostasis.178 Further studies using a nanocomposite composed of doxorubicin (chemotherapeutic), indocyanine green (sonosensitizer), and hyaluronic acid (CD44 targeting) demonstrated similar decreases in pulmonary metastasis.170 ROS production was also confirmed within the SDT group, potentially implicating its role in the observed antimetastatic effects. Altogether, while more preclinical models are necessary to establish the use of SDT as an anti-metastatic treatment, there does appear to exist preliminary preclinical evidence to support the application of SDT as an adjuvant therapy that can potentially prevent the onset of metastatic dissemination. The reduction in tumor metastasis observed following SDT may be the result of a cancer cell antigen-mediated immune response. As such, the concept of an SDT-induced immune response has been recently studied for SDT/immunotherapy.146,179 In a recent 2019 study, Yue et al. investigated nanoparticles comprising HMME as the sonosensitizer and imiquimod as a TLR7 agonist (Figure 5d). Intracellular ROS generation was confirmed following the application of SDT to 4T1 cells. The subsequent immune response elicited in vitro and in vivo was mildly enhanced acutely compared to SDT without imiquimod, as measured through cytokine secretion, DC maturation, and calreticulin (CRT) exposure. Crucially, no notable cytokine storm effects (a major toxic limitation of immunotherapy) were observed. The authors combined the imiquimod/SDT combination therapy with anti-PDL1 therapy in vivo in both a local 4T1 and polyclonal whole-body metastatic model (fLuc-4T1). In both, the SDT/PDL1 therapy was able to decrease primary and distant tumor growth in addition to tumor metastasis beyond PDL1 therapy alone. However, it was unclear whether imiquimod coencapsulation alongside HMME was necessary to mediate these therapeutic effects. Nevertheless, the immunomodulatory and therapeutic effects observed, including the induction of cytokines (TFN-α, IFN-γ) from TEM CD8+ T cell phenotype expression, suggests that combination SDT/immunotherapy is a promising avenue to explore that can capitalize on the coloading of immunomodulatory agents and sonosensitizers. This offers great potential as a multifunctional nanoagent for not just efficacious cytotoxic effects, but also secondary preventative effects. In addition to chemo/SDT and immune/SDT, SDT combination with gene therapy,162 photodynamic therapy,180,181 photothermal therapy,165,166,172 chemodynamic therapy,152 and radiotherapy171 have also been explored, yielding a plethora of multifunctional theranostic nanosonosensitizer agents. For example, Shanei et al. used gold nanoparticles to deliver single-agent SDT (1 MHz, 0.5/1/1.5 W/cm 2) and radiotherapy (X-ray, 0.5/1/2 Gy). 171 A significant decrease in cell viability was observed when combination therapy was delivered compared to ultrasound and radiotherapy alone. While the effect of SDT alone was not shown, a reduction of cell survival of 94.9% in combination is (particles containing Ce6 and TPZ). A similar technique of activating hypoxic prodrugs with SDT was employed by Feng et al., who loaded TPZ into mesoporous titanium nanoparticles modified by S-nitrosothiol.157 Akin to the above study, significant improvements in tumor inhibition were observed with the addition of the hypoxic prodrug. Alternative strategies in amplifying ROS generation revolve around targeting glutathione (GSH) levels within the tumor microenvironment. The free thiol groups in GSH function to protect cells against free-radical damage,173 thus compromising the therapeutic efficacy of ROS-based treatments such as SDT. Furthermore, GSH also holds the potential to be exploited for controlled drug release owing to its elevated concentration in tumor cells.174 Accordingly, the depletion and exploitation of GSH levels have been recently studied to enhance the efficacy of SDT-based treatment.152 For example, manganese-based nanocomposites were explored to both deplete GSH and generate O2 through H2O2 consumption to overcome the hypoxic tumor microenvironment. Fu et al. explored PEGylated K2FeO4 loaded onto hollow mesoporous organosilica nanoparticles (HMON) to which PpIX (sonosensitizer) was anchored with lauric acid (US-triggered phase change prodrug platform).56 The authors hoped the inclusion of iron would potentially enhance ·OH generation via the Fenton reaction, should that be an underlying mechanism behind SDT.162 Indeed, the iron oxide inclusion increased both 1O2 and OH· generation, confirmed through fluorescent ROS probes. A synergistic decrease of cell viability following SDT treatment (1 MHz, 2 W/cm2) was observed in both hypoxic and normoxic conditions in both a concentration-dependent and ultrasound intensity-dependent manner. This translated into the observation of tumor growth arrest over 18 days only when SDT was mediated with nanoparticles comprising the iron oxide and sonosensitizer. Similar manganese-based nanocomposites explored include PEGylated oxygen-deficient manganese−tungsten bimetallic nanoparticles for multimodal image-guided enhanced efficacy SDT, 175 and HMME (sonosensitizer)-Acriflavine (inhibitor of HIF-1α) encapsulated liposomes coated with MnO2 nanosheets and decorated with AS1411 aptamer for tumor targeting.176 Thus far, strategies to overcome or exploit tumor hypoxia to enhance SDT effects are predominantly based on the coloading of oxygen or hypoxic prodrugs alongside sonosensitizers within nanoformulations. In addition, autophagy inhibitors have also been employed to induce vessel-normalization,140 and remodeling of tumor-associated macrophage phenotype (M2 → M1).177 These approaches toward amplifying ROS generation have only recently been implemented and thus may warrant further exploration, including greater characterization of downstream effects and any potential resistance arising from activation of alternative antioxidant pathways, such as Trx and NRF2. 3.2.4. Combination Therapies Derived from Multifunctionality. The codelivery of sonosensitizers and hypoxia agents can be extended to the multifunctional formulation of nanomedicines containing sensitizers and chemotherapeutics for synergistic or combination therapy. As overviewed above, coloading of doxorubicin and sonosensitizers within a single nanoparticle can facilitate on-demand, externally stimulated drug delivery. This has since been applied using different sonosensitizer−doxorubicin combinations163,147 and extended to the SDT-stimulated delivery of paclitaxel167 and curcumin.169 Beyond its use to trigger drug delivery, chemo/SDT N https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review pressure reporting, while also combining in solution detection of soluminescence, broadband emissions associated with inertial cavitation and ROS generation to characterize their SDT platform.120,183 The correlation of acoustic emissions in vivo with SDT bioeffects, though more challenging, can be guided by existing methodologies implemented in the field of microbubble-enabled blood-brain barrier disruption summarized recently by Jones and Hynynen.184 4.1.3. Translation of in Vitro Mechanisms to an in Vivo Setting. When possible, the above techniques should be applied both in vitro and in vivo. Although in vitro mechanistic studies evaluating ROS generation have informed our understanding of SDT, they are not able to account for the influence of the complex tumor microenvironment, interstitial and solid tumor pressures, three-dimensional volumetric boundaries for bubble cavitation, ROS quenchers in tissue, and light absorbers on cavitational bioeffects. Thus, in vivo probing of sonosensitizer biodistribution, inertial cavitation, ROS generation, and sonoluminescence would be valuable in establishing mechanisms of action. In vivo biodistribution studies could particularly facilitate delineation between bioeffects from enhanced drug delivery versus those that are truly sonodynamic in nature. Specifically, biodistribution of free sensitizers should be compared to that associated with nanoformulations to truly differentiate any drug delivery advantages of nanomedicines. These biodistribution studies should be performed quanitatively via analytical techniques such as high-performance liquid chromatography, spectrofluorometry, or inductively coupled mass spectrometry quantification of sonosensitizers extracted from tissue, or through radioisotope-enabled quatitative methods such as positron emission tomography or γ-counting of tissues. In order to more comprehensively assess whether the bioeffects observed are a result of cavitation-mediated vasculature disruption rather than the sonodynamic activation of sonosensitizers, techniques that assess tumor vascularity can be employed, such as gadolinium-enhanced T1w-MRI. These techniques should be combined with sonosensitizer biodistribution analysis across appropriate control groups as will be discussed below. We do note that the translation of in vitro characterization of sonoluminescence and ROS generation to an in vivo setting is challenging. However, we hope that transdisciplinary collaborations among chemists, physicists, histologists, and biologists may generate new in vivo probes and imaging paradigms to address this hurdle. For example, advances in pairing intravital imaging with ultrasound delivery, cavitation detection, and the development of novel fluorescent and chemiluminescent probes may be of value in garnering SDT mechanistic insights in vivo. Furthermore, acute histological analysis of tumor tissue could further help distinguish bioeffects and resulting mechanisms of action, including differentiation of expected SDT cell apoptosis from thermally induced tissue necrosis and histotripsy tissue fractionation. 4.1.4. Use of Appropriate Controls. The current broad definition of SDT involves the use of low frequency ultrasound to activate therapeutic effects of sonosensitizers. Though synergy between ultrasound and sonosensitizer is often implied, this synergy can only be demonstrated by incorporating adequate controls into therapeutic studies. Specifically, this broad definition and implication of synergy necessitates at minimum the comparison of therapeutic effects associated with (i) ultrasound alone, (ii) sonosensitizer alone, and (iii) nonetheless highly promising. Furthermore, a SDT/radiation therapy strategy can capitalize upon existing platforms that have already been explored clinically to deliver HIFU therapy in conjunction with low-dose radiotherapy.182 Indeed, all of the above explored SDT combination therapy paradigms can benefit from advances in the implementation of individual therapies, leading to potentially synergistic effects that can augment the therapeutic capacity of SDT. 4. FUTURE OUTLOOK 4.1. Overcoming Current Limitations. Despite the advantages that nanomedicine has brought to SDT, ambiguity surrounding the mechanisms of action at play creates limitations in maximizing therapeutic efficacy. This is further challenged by a lack of standardization in ultrasound dosimetry and a unifying definition of SDT. To overcome these challenges, we propose reporting acoustic intensities in the form of pressures, assessing ultrasound acoustic emissions during SDT, detecting sonoluminescence, quantifying sensitizer biodistribution, assessing tumor vasculature permeability, and employing appropriate treatment controls as discussed below. 4.1.1. Standard Reporting of Ultrasound Parameters. As discussed, cavitational bioeffects depend largely on acoustic pressure. It is therefore peculiar that acoustic pressures are rarely reported in SDT studies. Instead, authors typically report acoustic intensities in the form of power density (W/cm2). This limits the comparisons that can be made between studies and does not provide enough context to delineate whether the pressure pulses fall within realms of stable or inertial cavitation. While pressure−intensity conversions may be estimated using the formula I = p2/ρc, where I = instantaneous acoustic intensity, p = root-mean-square pressure amplitude, ρ = density of the propagating medium, and c = velocity of sound in the propagating medium, significant assumptions must be made to derive pressures in this manner since each ultrasound transducer’s output power density corresponds to differing pressures. As such, authors should ensure that they calibrate their ultrasound systems and transducers to more accurately report acoustic intensity in the form of pressure, and outline their ultrasound setups and beam characteristics in more detail. We hope that the combined reporting of frequency, beam geometry, ultrasound setup, and pressure becomes standard so that lessons learned from individual studies can be more broadly applied. 4.1.2. Better Characterization of Cavitation and Resultant Effects. As ROS generation is the predominating therapeutic mechanism of action used to explain SDT effects, there exists an underlying assumption that inertial cavitation is a prerequisite of SDT. However, few studies directly provide evidence of inertial cavitation, or any resulting hotspot temperature changes and sonoluminescence. In order to better elucidate SDT mechanisms, acoustic cavitation, sonoluminescence, and temperature detection within SDT studies would be of great value. This is particularly vital in evaluating the therapeutic utility of nanoparticles in SDT: cavitation detection over a range of pressures will better differentiate whether nanoparticles augment SDT effects by lowering cavitation thresholds as exogenous sources of cavitation nuclei, or by other mechanistic means such as improving sonosensitizer bioavailability or activity. To this end, lessons can be learned from recent studies by Beguin et al., who thoroughly described their acoustic instrumentation, including acoustic O https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review include nanobubbles, echogenic liposomes, gas vesicles, and cavitation seeds.187 Echogenic liposomes comprising gas pockets within the lipid bilayer or core monolayers may particularly be of interest, as they may facilitate higher sensitizer loading versus nanobubbles or droplets.187,188 The above submicron SDT strategies are advantageous for tumors displaying the enhanced permeability and retention (EPR) effect, whereby the rapid growth of tumors leads to leaky vasculature, allowing nanoparticle extravasation followed by retention due to impaired lymphatic drainage. Tumors that are not susceptible to the EPR effect, such as blood-brain barrier intact glioblastomas, would require alternative strategies not reliant on the EPR effect for nanomedicine delivery. One such example is the use of an acoustic microbubble-tonanobubble conversion, introduced by Hunyh et al.189 Here, bacteriochlorophyll−lipid shelled microbubbles underwent an in situ conversion into nanoparticles following the application of low frequency therapeutic ultrasound. This led to early sequestration of the resulting daughter nanoparticles within KB subcutaneous tumors in mice, potentially from vasculature disruption at the tumor site. Electron microscope imaging indicated that this conversion likely produced nanobubbles as opposed to gas-devoid nanoparticles. Presumably, this microto-nano conversion could be exploited to deliver nanobubbles across intact tumor vasculature, where the bubbles could then be stimulated to cavitate and activate the loaded sonosensitizer (in this case, bacteriochlorophyll, a sister compound to porphyrin) for extravascular SDT. However, prior to realizing this potential, confirmation of vasculature disruption and the delivery of nanobubbles through this micro-to-nano conversion is required. 4.3. Bearings on Clinical Translation. The ultimate goal of improving and optimizing any therapeutic paradigm preclinically, including SDT, is translation to the clinic. Despite the myriad in vivo SDT studies, clinical trial validation remains to be sought. While clinical case studies report initial promise,190,191 only combination therapy with PDT180 or immunotherapy has been explored for cancer treatment, making it difficult to elucidate the true independent clinical effects of SDT. The largest of such studies featured 115 advanced metastatic patients unresponsive to traditional therapy with varying primary tumor sites.180 These patients received combinatorial sono-photodynamic therapy (1 MHz, 1 W/cm2) with Sonnelux-1 under generalized light and showed an overall increase of predicted median survival. Although preliminarily promising, it remains difficult to evaluate the study in depth given the lack of statistical analysis conducted with regard to overall survival benefit and a selective representation of patient data in favor of those that showed improvement to predicted median survival. Furthermore, no information regarding the ultrasound apparatus was provided. Given the usage of ultrasonic irradiation in a bathtub as well as other suboptimal unfocused ultrasound platforms for SDT treatment in previous case studies,190 this lack of information is troubling. The limited clinical application of SDT is unsurprising. The SDT field is still young and, as discussed, requires further exploration before realizing its promising clinical potential as a minimally invasive, safe, and targeted adjunctive cancer therapy. This limited clinical exploration underscores the need to develop a greater understanding of the mechanisms underlying SDT, but also more detailed exploration beyond proof-of-concept studies of existing therapeutic regimens that combination of ultrasound and sonosensitizer. Additionally, synergy should be calculated and not assumed in order to distinguish from additive effects. This is particularly of importance when assessing SDT in combination with other therapies, such as PDT, chemotherapy, PTT, and others. The implementation of ultrasound-only controls when combined with tumor vessel permeability assessment and thermometry will additionally allow the contribution of thermal ablation, hyperthermia, and vasculature disruption toward observed bioeffects to be better assessed. To this end, MRI thermometry during SDT can provide valuable information as evidenced by Wu et al.35 Furthermore, the implementation of micron and nanosized agents in SDT additionally requires the comparison of free and encapsulated sonosensitizer with and without ultrasound administration to truly realize the therapeutic beneficence of using supramolecular variants of sonosensitizers. Lastly, given the debate over whether sonosensitizers function as photodynamic agents, amplifiers of pyrolysis, or mediators of mechanical bioeffects, the photodynamic activity of sonosensitizers should be characterized if unknown. Together, these controls can better differentiate bioeffects resulting from microbubble cavitation versus sonosensitizer activation. 4.1.5. Generating Sensitizer-Specific Structure/Activity Relationships. Collectively, the application of the above recommendations across different classes of sensitizers may better clarify SDT structure/activity relationships. We hope these studies reevaluate seminal disseminations and previously studied sensitizers using advances in ROS probe chemistry, acoustic cavitation detection, and ultrasound transducer design to better clarify past contradicting evidence surrounding SDT mechanisms of action. This demanding task would allow better optimization of sonosensitizers, elucidation of nanomedicine benefits, and more informed selection of synergistic therapy paradigms. 4.2. Expanding the Unique Role of Nanomedicine. The established utility of nanomedicine in SDT, as overviewed above, can be further expanded through the use of submicron ultrasound contrast agents. These nanosized echogenic particles may function similarly to microbubbles as exogenously delivered acoustically responsive nuclei that may lower the threshold for acoustic cavitation required for SDT. Unlike limitations faced by their micron-sized counterparts, nanosized contrast agents have the ability to extravasate out of leaky tumor vasculature into the tumor interstitium, with optimal nanoparticle sizes for passive tumor uptake thought to be in the 100−150 nm size range.185,186 This could allow for more efficient SDT to be conducted within both the intravascular and extravascular tumor spaces, expanding the therapeutic horizons for SDT and allowing for spatially varied SDT effects to be better studied, a feat unachievable with micron-sized contrast agents. The potential of sensitizer-loaded, nanosized ultrasound contrast agents to act as SDT sensitizers remains untapped, with merely a single in vivo160 study published in 2019 to the best of our knowledge that made use of echogenic nanoparticles. In this study, nanodroplets were used to deliver IR780 sensitizer beyond the tumor vasculature and incite SDT in 4T1 tumor-bearing mice. Thus, there is much to still be learned about the use of nanosized ultrasound contrast agents as extravascular agents, including whether they can lower the cavitation threshold of SDT, induce more potent effects versus nonechogenic nanoparticles, deliver extravascular SDT, and extend to echogenic nanoparticles beyond nanodroplets to P https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review field in establishing universal mechanisms of SDT, innovation continues in the field. Promoted by advances in nanotechnology, numerous novel nanoplatforms have been developed to better enhance the therapeutic efficacy of SDT. Through enhanced spatiotemporal accumulation of single or multiple drug agents in high packing densities combined with their ability to function as cavitation nuclei, nanoscaled sonosensitizers may overcome the barriers observed thus far in achieving therapeutically effective SDT. Thus, when combined with advances in the clinical implementation of low frequency focused ultrasound, and better mechanistic clarity, nanomedicines may bring the field of SDT closer to realizing its exciting therapeutic potential. have already shown initial preclinical success. To better illuminate the necessity of establishing more detailed mechanistic understanding, one needs only to look within this decade toward latrepirdine, an antihistamine proposed for usage in Alzheimer’s disease. Similar to SDT, preclinical studies showed very promising results in vitro, although no investigation was undertaken to comprehensively evaluate the mechanism by which such effects were exerted.192 This inevitably proved to be its downfall, as three pivotal phase III clinical trials all showed negligible cognitive benefits, and only recently has data contradicted previously held hypotheses on latrepirdine mechanistics.193 It is true that for a drug to gain FDA approval, understanding of mechanisms is not strictly required, and indeed, the mechanisms of action of many highly prescribed drugs remain unknown. Nevertheless, this knowledge, particularly when considering a novel therapeutic modality such as SDT, guides drug development and is essential in providing direction by which the field may grow. As such, the formation of a foundational understanding of SDT will be the first step toward its effective clinical translation. In understanding SDT, the field has a greater chance of overcoming hurdles in delivering effective SDT preclinically, without which clinical trial of SDT is inherently impeded. Once consistent, effective SDT is achieved preclinically; its successive clinical translation will then also depend on its rational application to appropriate cancer lesions. SDT translation will also require advances in the clinical implementation of therapeutic focused ultrasound, including MRI-guidance and more readily available MRI and patientcompatible focused ultrasound transducers. Finally, the success of clinical SDT will also be contingent on the effective translation of sonosensitizers and sonosensitive nanomedicines. Thus, intrinsically, the challenging task of realizing the therapeutic potential of SDT will require collaboration across multiple disciplines. ■ AUTHOR INFORMATION Corresponding Author Gang Zheng − Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada M5G 1L7; Institute of Biomaterials and Biomedical Engineering and Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada M5S 3G9; orcid.org/00000002-0705-7398; Email: gang.zheng@uhnres.utoronto.ca Authors Victor Choi − Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada M5G 1L7; School of Pharmacy, University College London, London, United Kingdom WC1N 1AX Maneesha A. Rajora − Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada M5G 1L7; Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, Ontario, Canada M5S 3G9 Complete contact information is available at: https://pubs.acs.org/10.1021/acs.bioconjchem.0c00029 Author Contributions 5. CONCLUSIONS It is well-known that first-line and adjunct treatment modalities for cancer, including chemotherapy, ionizing radiation, and surgical resection, are invasive, immunosuppressive, or cumulatively toxic. Therefore, the promise of a new therapeutic option that can access and treat deep-seated tumor lesions in a minimally invasive manner using relatively nontoxic agents warrants the broader exploration of SDT as a cancer therapy. While a growing amount of research has been conducted preclinically, a deeper understanding of the mechanisms underlying SDT is needed for the field to realize its potential. These mechanisms include mechanical cell membrane shearing and ROS-mediated cytotoxicity from pyrolysis or sonoluminescence, wherein ultrasound-triggered, sonosensitizer-enabled ROS generation currently stands as the prevailing proposed mechanism of action. These are all plausible contributors to therapeutic efficacy, whereby the predominating mechanism is likely dependent on the physiochemical properties of sonosensitizers, ultrasound dosimetry, and sensitizer intratumoral and subcellular localization. While it is unreasonable to expect a single comprehensive study analyzing each combination of these variables, we hope that standard reporting and characterization of ultrasound dosimetry, inertial versus stable cavitation, in vivo mechanistic studies, and the use of appropriate therapeutic controls can generate transferrable knowledge to better inform the design and implementation of SDT paradigms. Nevertheless, despite challenges faced by the # This manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. V.C. and M.A.R. contributed equally. Notes The authors declare no competing financial interest. ■ ACKNOWLEDGMENTS The authors would like to thank Alex Dhaliwal and Carly Pellow for their technical expertise. The authors acknowledge their funding sources: the Terry Fox Research Institute, the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Princess Margaret Cancer Foundation, Canada Research Chairs Program and the McLaughlin Centre. ■ ABBREVIATIONS CMD, carboxymethyl dextran; DOX, doxorubicin; DVDMS, sinoporphyrin sodium; EPR, enhanced permeability and retention; GSH, glutathione; HIF-1α, hypoxia inducible factor 1-alpha; HIFU, high intensity focused ultrasound; HMME, hematoporphyrin monomethyl ether; HMONs, hollow mesoporous organosilica nanoparticles; IFN-γ, interferon gamma; MDRP1, multidrug resistance protein 1; MMP, mitochondrial membrane potential; MRI, magnetic resonance imaging; MSN, mesoporous silica nanoparticle; NRF2, nuclear factor erythroid 2-related factor 2; NSAIDs, nonsteroidal anti-inflammatory Q https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc (16) Huang, Z. (2005) A Review of Progress in Clinical Photodynamic Therapy. Technol. Cancer Res. Treat. 4, 283−293. (17) Suslick, K. S. (1990) Sonochemistry. Science 247, 1439. (18) Umemura, S., Yumita, N., and Nishigaki, R. (1993) Enhancement of Ultrasonically Induced Cell Damage by a GalliumPorphyrin Complex, Atx-70. Jpn. J. Cancer Res. 84, 582−588. (19) Kim, K.-H., Kim, J.-K., and Lee, D.-H. (2011) Sonodynamic Induced Antitumor Effect of Radachlorin on Solid Tumor. Curr. Appl. Phys. 11, 559−563. (20) Sato, K., Hachino, K., and Saito, Y. (2004) Inception and Dynamics of Traveling-Bubble-Type Cavitation in a Venturi. Nippon Kikai Gakkai Ronbunshu, B-hen 70, 69−76. (21) Krasovitski, B., Frenkel, V., Shoham, S., and Kimmel, E. (2011) Intramembrane Cavitation as a Unifying Mechanism for UltrasoundInduced Bioeffects. Proc. Natl. Acad. Sci. U. S. A. 108, 3258−3263. (22) Church, C. C., Labuda, C., and Nightingale, K. (2015) A Theoretical Study of Inertial Cavitation from Acoustic Radiation Force Impulse Imaging and Implications for the Mechanical Index. Ultrasound Med. Biol. 41, 472−485. (23) Misik, V., Miyoshi, N., and Riesz, P. (1995) Epr Spin-Trapping Study of the Sonolysis of H2o/D2o Mixtures: Probing the Temperatures of Cavitation Regions. J. Phys. Chem. 99, 3605−3611. (24) Suslick, K. S., Hammerton, D. A., and Cline, R. E. (1986) Sonochemical Hot Spot. J. Am. Chem. Soc. 108, 5641−5642. (25) Didenko, Y. T., McNamara, W. B., and Suslick, K. S. (1999) Hot Spot Conditions During Cavitation in Water. J. Am. Chem. Soc. 121, 5817−5818. (26) Suslick, K. S., and Flannigan, D. J. (2008) Inside a Collapsing Bubble: Sonoluminescence and the Conditions During Cavitation. Annu. Rev. Phys. Chem. 59, 659−683. (27) Rayleigh, L. (1917) Viii. On the Pressure Developed in a Liquid During the Collapse of a Spherical Cavity. Philos. Mag. 34, 94− 98. (28) Frenzel, H., and Schultes, H. (1934) Luminescenz Im Ultraschallbeschickten Wasser. Z. Phys. Chem. 27B, 421. (29) E Apfel, R. (1982) Acoustic Cavitation: A Possible Consequence of Biomedical Uses of Ultrasound. Br. J. Cancer Suppl. 5, 140−146. (30) Gogate, P. R., and Kabadi, A. M. (2009) A Review of Applications of Cavitation in Biochemical Engineering/Biotechnology. Biochem. Eng. J. 44, 60−72. (31) Prosperetti, A., Crum, L. A., and Commander, K. W. (1988) Nonlinear Bubble Dynamics. J. Acoust. Soc. Am. 83, 502−514. (32) O’Brien, W. D., Jr (1986) Biological Effects of Ultrasound: Rationale for the Measurement of Selected Ultrasonic Output Quantities. Echocardiography 3, 165−179. (33) Hiller, R. A., Putterman, S. J., and Weninger, K. R. (1998) Time-Resolved Spectra of Sonoluminescence. Phys. Rev. Lett. 80, 1090−1093. (34) Riesz, P., Berdahl, D., and Christman, C. L. (1985) Free Radical Generation by Ultrasound in Aqueous and Nonaqueous Solutions. Environ. Health Perspect. 64, 233−252. (35) Wu, S. K., Santos, M. A., Marcus, S. L., and Hynynen, K. (2019) Mr-Guided Focused Ultrasound Facilitates Sonodynamic Therapy with 5-Aminolevulinic Acid in a Rat Glioma Model. Sci. Rep. 9, 10465. (36) Yoshida, M., Kobayashi, H., Terasaka, S., Endo, S., Yamaguchi, S., Motegi, H., Itay, R., Suzuki, S., Brokman, O., Shapira, Y., et al. (2019) Sonodynamic Therapy for Malignant Glioma Using 220-Khz Transcranial Magnetic Resonance Imaging-Guided Focused Ultrasound and 5-Aminolevulinic Acid. Ultrasound Med. Biol. 45, 526−538. (37) Maxwell, A. D., Cain, C. A., Duryea, A. P., Yuan, L., Gurm, H. S., and Xu, Z. (2009) Noninvasive Thrombolysis Using Pulsed Ultrasound Cavitation Therapy - Histotripsy. Ultrasound Med. Biol. 35, 1982−1994. (38) Bader, K. B., Vlaisavljevich, E., and Maxwell, A. D. (2019) For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy. Ultrasound Med. Biol. 45, 1056−1080. drugs; PCMS, porphyrin-like metal centers; PD-L1, programmed death-ligand 1; PDT, photodynamic therapy; PEG, polyethylene glycol; PFC, perfluorocarbon; PFP, perfluoropropane; PpIX, protoporphyrin IX; PTFE, polytetrafluoroethylene; PTX, paclitaxel; RB, Rose Bengal; ROS, reactive oxygen species; RT, radiotherapy; SDT, sonodynamic therapy; TAPP, tetra-(4-aminophenyl) porphyrin; TLR7, toll-like receptor 7; Trx, thioredoxin reductase; TPZ, tirapazamine ■ Review REFERENCES (1) Mitragotri, S. (2005) Healing Sound: The Use of Ultrasound in Drug Delivery and Other Therapeutic Applications. Nat. Rev. Drug Discovery 4, 255−260. (2) Bailey, M. R., Khokhlova, V. A., Sapozhnikov, O. A., Kargl, S. G., and Crum, L. A. (2003) Physical Mechanisms of the Therapeutic Effect of Ultrasound (a Review). Acoust. Phys. 49, 369−388. (3) van den Bijgaart, R. J. E., Eikelenboom, D. C., Hoogenboom, M., Fütterer, J. J., den Brok, M. H., and Adema, G. J. (2017) Thermal and Mechanical High-Intensity Focused Ultrasound: Perspectives on Tumor Ablation, Immune Effects and Combination Strategies. Cancer Immunol. Immunother. 66, 247−258. (4) Roberts, W. W., Hall, T. L., Ives, K., Wolf, J. S., Jr, Fowlkes, J. B., and Cain, C. A. (2006) Pulsed Cavitational Ultrasound: A Noninvasive Technology for Controlled Tissue Ablation (Histotripsy) in the Rabbit Kidney. J. Urol. 175, 734−738. (5) Poon, C., McMahon, D., and Hynynen, K. (2017) Noninvasive and Targeted Delivery of Therapeutics to the Brain Using Focused Ultrasound. Neuropharmacology 120, 20−37. (6) Burgess, A., and Hynynen, K. (2013) Noninvasive and Targeted Drug Delivery to the Brain Using Focused Ultrasound. ACS Chem. Neurosci. 4, 519−526. (7) McMahon, D., Poon, C., and Hynynen, K. (2019) Evaluating the Safety Profile of Focused Ultrasound and Microbubble-Mediated Treatments to Increase Blood-Brain Barrier Permeability. Expert Opin. Drug Delivery 16, 129−142. (8) Mainprize, T., Lipsman, N., Huang, Y., Meng, Y., Bethune, A., Ironside, S., Heyn, C., Alkins, R., Trudeau, M., Sahgal, A., et al. (2019) Blood-Brain Barrier Opening in Primary Brain Tumors with Non-Invasive Mr-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 9, 321. (9) Kotopoulis, S., Delalande, A., Popa, M., Mamaeva, V., Dimcevski, G., Gilja, O. H., Postema, M., Gjertsen, B. T., and McCormack, E. (2014) Sonoporation-Enhanced Chemotherapy Significantly Reduces Primary Tumour Burden in an Orthotopic Pancreatic Cancer Xenograft. Mol. Imaging Biol. 16, 53−62. (10) Di Ianni, T., Bose, R. J. C., Sukumar, U. K., Bachawal, S., Wang, H., Telichko, A., Herickhoff, C., Robinson, E., Baker, S., VilchesMoure, J. G., et al. (2019) Ultrasound/Microbubble-Mediated Targeted Delivery of Anticancer Microrna-Loaded Nanoparticles to Deep Tissues in Pigs. J. Controlled Release 309, 1−10. (11) Ferrara, K., Pollard, R., and Borden, M. (2007) Ultrasound Microbubble Contrast Agents: Fundamentals and Application to Gene and Drug Delivery. Annu. Rev. Biomed. Eng. 9, 415−447. (12) Yumita, N., Nishigaki, R., Umemura, K., and Umemura, S. (1991) Sonodynamic Therapy. Antitumor Activation of Drug by Ultrasound. Japanese Journal of Medical Ultrasonics 18, 1−9. (13) Castano, A. P., Mroz, P., and Hamblin, M. R. (2006) Photodynamic Therapy and Anti-Tumour Immunity. Nat. Rev. Cancer 6, 535−545. (14) McEwan, C., Nesbitt, H., Nicholas, D., Kavanagh, O. N., McKenna, K., Loan, P., Jack, I. G., McHale, A. P., and Callan, J. F. (2016) Comparing the Efficacy of Photodynamic and Sonodynamic Therapy in Non-Melanoma and Melanoma Skin Cancer. Bioorg. Med. Chem. 24, 3023−3028. (15) Rosenthal, I., Sostaric, J. Z., and Riesz, P. (2004) Sonodynamic Therapy–a Review of the Synergistic Effects of Drugs and Ultrasound. Ultrason. Sonochem. 11, 349−363. R https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc (39) Castano, A. P., Demidova, T. N., and Hamblin, M. R. (2004) Mechanisms in Photodynamic Therapy: Part One-Photosensitizers, Photochemistry and Cellular Localization. Photodiagn. Photodyn. Ther. 1, 279−293. (40) Tang, W., Liu, Q., Wang, X., Wang, P., Zhang, J., and Cao, B. (2009) Potential Mechanism in Sonodynamic Therapy and Focused Ultrasound Induced Apoptosis in Sarcoma 180 Cells in Vitro. Ultrasonics 49, 786−793. (41) He, Y., Xing, D., Yan, G., and Ueda, K.-i. (2002) Fcla Chemiluminescence from Sonodynamic Action in Vitro and in Vivo. Cancer Lett. 182, 141−145. (42) Yumita, N., Iwase, Y., Nishi, K., Komatsu, H., Takeda, K., Onodera, K., Fukai, T., Ikeda, T., Umemura, S.-I., Okudaira, K., et al. (2012) Involvement of Reactive Oxygen Species in Sonodynamically Induced Apoptosis Using a Novel Porphyrin Derivative. Theranostics 2, 880−888. (43) Wang, H., Yang, Y., Chen, H., Dan, J., Cheng, J., Guo, S., Sun, X., Wang, W., Ai, Y., Li, S., et al. (2014) The Predominant Pathway of Apoptosis in Thp-1 Macrophage-Derived Foam Cells Induced by 5Aminolevulinic Acid-Mediated Sonodynamic Therapy Is the Mitochondria-Caspase Pathway Despite the Participation of Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 33, 1789−1801. (44) Zheng, L., Sun, X., Zhu, X., Lv, F., Zhong, Z., Zhang, F., Guo, W., Cao, W., Yang, L., and Tian, Y. (2014) Apoptosis of Thp-1 Derived Macrophages Induced by Sonodynamic Therapy Using a New Sonosensitizer Hydroxyl Acetylated Curcumin. PLoS One 9, No. e93133. (45) Wang, P., Wang, X., Zhang, K., Gao, K., Song, M., and Liu, Q. (2013) The Spectroscopy Analyses of Ppix by Ultrasound Irradiation and Its Sonotoxicity in Vitro. Ultrasonics 53, 935−942. (46) Yumita, N., and Umemura, S.-i. (2004) Ultrasonically Induced Cell Damage and Membrane Lipid Peroxidation by Photofrin Ii: Mechanism of Sonodynamic Activation. J. Med. Ultrason. 31, 35−40. (47) Umemura, S.-i., Yumita, N., Umemura, K., and Nishigaki, R. (1999) Sonodynamically Induced Effect of Rose Bengal on Isolated Sarcoma 180 Cells. Cancer Chemother. Pharmacol. 43, 389−393. (48) Yumita, N., Kawabata, K.-i., Sasaki, K., and Umemura, S.-i. (2002) Sonodynamic Effect of Erythrosin B on Sarcoma 180 Cells in Vitro. Ultrason. Sonochem. 9, 259−265. (49) Okada, K., Itoi, E., Miyakoshi, N., Nakajima, M., Suzuki, T., and Nishida, J. (2002) Enhanced Antitumor Effect of Ultrasound in the Presence of Piroxicam in a Mouse Air Pouch Model. Jpn. J. Cancer Res. 93, 216−222. (50) Umemura, K., Yumita, N., Nishigaki, R., and Umemura, S.-i. (1996) Sonodynamically Induced Antitumor Effect of Pheophorbide A. Cancer Lett. 102, 151−157. (51) Szabó, L., Tóth, T., Rácz, G., Takács, E., and Wojnárovits, L. (2016) Drugs with Susceptible Sites for Free Radical Induced Oxidative Transformations: The Case of a Penicillin. Free Radical Res. 50, 26−38. (52) Mišík, V., Miyoshi, N., and Riesz, P. (1996) Epr Spin Trapping Study of the Decomposition of Azo Compounds in Aqueous Solutions by Ultrasound: Potential for Use as Sonodynamic Sensitizers for Cell Killing. Free Radical Res. 25, 13−22. (53) Itano, O., Onishi, T., Matsuda, S., Fujimura, T., Jinno, H., Kitagou, M., Shinoda, M., Kawachi, S., Tanabe, M., Ogino, C., et al. (2012) A Novel Sonodynamic Therapy Using Hydroxyl Radical Generated from Ultrasound Activated Tio2; for Human Epithelial Carcinoma Cells. Cancer Res. 72, 4341. (54) Cheng, J., Sun, X., Guo, S., Cao, W., Chen, H., Jin, Y., Li, B., Li, Q., Wang, H., Wang, Z., et al. (2013) Effects of 5-Aminolevulinic Acid-Mediated Sonodynamic Therapy on Macrophages. Int. J. Nanomed. 8, 669−676. (55) Guo, S., Sun, X., Cheng, J., Xu, H., Dan, J., Shen, J., Zhou, Q., Zhang, Y., Meng, L., Cao, W., et al. (2013) Apoptosis of Thp-1 Macrophages Induced by Protoporphyrin Ix-Mediated Sonodynamic Therapy. Int. J. Nanomed. 8, 2239−2246. (56) Fu, J., Li, T., Zhu, Y., and Hao, Y. (2019) Ultrasound-Activated Oxygen and Ros Generation Nanosystem Systematically Modulates Review Tumor Microenvironment and Sensitizes Sonodynamic Therapy for Hypoxic Solid Tumors. Adv. Funct. Mater. 29, 1906195. (57) Shen, S., Wu, L., Liu, J., Xie, M., Shen, H., Qi, X., Yan, Y., Ge, Y., and Jin, Y. (2015) Core-Shell Structured Fe3o4@Tio2Doxorubicin Nanoparticles for Targeted Chemo-Sonodynamic Therapy of Cancer. Int. J. Pharm. 486, 380−388. (58) Simon, H. U., Haj-Yehia, A., and Levi-Schaffer, F. (2000) Role of Reactive Oxygen Species (ROS) in Apoptosis Induction. Apoptosis 5, 415−418. (59) Cadet, J., Douki, T., and Ravanat, J.-L. (2008) Oxidatively Generated Damage to the Guanine Moiety of DNA: Mechanistic Aspects and Formation in Cells. Acc. Chem. Res. 41, 1075−1083. (60) Tachibana, K., Uchida, T., Ogawa, K., Yamashita, N., and Tamura, K. (1999) Induction of Cell-Membrane Porosity by Ultrasound. Lancet 353, 1409. (61) Tang, W., Liu, Q., Wang, X., Mi, N., Wang, P., and Zhang, J. (2008) Membrane Fluidity Altering and Enzyme Inactivating in Sarcoma 180 Cells Post the Exposure to Sonoactivated Hematoporphyrin in Vitro. Ultrasonics 48, 66−73. (62) Yumita, N., Umemura, S., Magario, N., Umemura, K., and Nishigaki, R. (1996) Membrane Lipid Peroxidation as a Mechanism of Sonodynamically Induced Erythrocyte Lysis. Int. J. Radiat. Biol. 69, 397−404. (63) Repetto, M. S. J., and Boveris, A. (2012) Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. In Lipid Peroxidation (Catala, A., Ed.), pp 3−30, Chapter 1, Vol. 1, InTech, London. (64) Rebillard, A., Tekpli, X., Meurette, O., Sergent, O., LeMoigneMuller, G., Vernhet, L., Gorria, M., Chevanne, M., Christmann, M., Kaina, B., et al. (2007) Cisplatin-Induced Apoptosis Involves Membrane Fluidification Via Inhibition of NHE1 in Human Colon Cancer Cells. Cancer Res. 67, 7865. (65) Grunicke, H., and Hofmann, J. (1992) Cytotoxic and Cytostatic Effects of Antitumor Agents Induced at the Plasma Membrane Level. Pharmacol. Ther. 55, 1−30. (66) Hueber, A.-O., Bernard, A.-M., Hérincs, Z., Couzinet, A., and He, H.-T. (2002) An Essential Role for Membrane Rafts in the Initiation of Fas/Cd95-Triggered Cell Death in Mouse Thymocytes. EMBO Rep. 3, 190−196. (67) Honda, H., Kondo, T., Zhao, Q.-L., Feril, L. B., and Kitagawa, H. (2004) Role of Intracellular Calcium Ions and Reactive Oxygen Species in Apoptosis Induced by Ultrasound. Ultrasound Med. Biol. 30, 683−692. (68) Heath-Engel, H. M., and Shore, G. C. (2006) Mitochondrial Membrane Dynamics, Cristae Remodelling and Apoptosis. Biochim. Biophys. Acta, Mol. Cell Res. 1763, 549−560. (69) Tang, W., Fan, W., Liu, Q., Zhang, J., and Qin, X. (2011) The Role of p53 in the Response of Tumor Cells to Sonodynamic Therapy in Vitro. Ultrasonics 51, 777−785. (70) Li, E., Sun, Y., Lv, G., Li, Y., Zhang, Z., Hu, Z., and Cao, W. (2019) Sinoporphyrin Sodium Based Sonodynamic Therapy Induces Anti-Tumor Effects in Hepatocellular Carcinoma and Activates p53/ Caspase 3 Axis. Int. J. Biochem. Cell Biol. 113, 104−114. (71) Levine, A. J. (1997) p53, the Cellular Gatekeeper for Growth and Division. Cell 88, 323−331. (72) Fischer, J. L., Mihelc, E. M., Pollok, K. E., and Smith, M. L. (2007) Chemotherapeutic Selectivity Conferred by Selenium: A Role for p53-Dependent DNA Repair. Mol. Cancer Ther. 6, 355. (73) Saksena, T. K., and Nyborg, W. L. (1970) Sonoluminescence from Stable Cavitation. J. Chem. Phys. 53, 1722−1734. (74) Gaitan, D. F., Crum, L. A., Church, C. C., and Roy, R. A. (1992) Sonoluminescence and Bubble Dynamics for a Single, Stable, Cavitation Bubble. J. Acoust. Soc. Am. 91, 3166−3183. (75) Leighton, T. G. (1994) Effects and Mechanisms. In The Acoustic Bubble (Leighton, T. G., Ed.) pp 439−590, Academic Press, New York. (76) Umemura, S.-i., Yumita, N., Nishigaki, R., and Umemura, K. (1990) Mechanism of Cell Damage by Ultrasound in Combination with Hematoporphyrin. Jpn. J. Cancer Res. 81, 962−966. S https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc (77) Mišík, V., and Riesz, P. (1996) Peroxyl Radical Formation in Aqueous Solutions of N,N-Dimethylformamide, N-Methylformamide, and Dimethylsulfoxide by Ultrasound: Implications for Sonosensitized Cell Killing. Free Radical Biol. Med. 20, 129−138. (78) Umemura, S., Yumita, N., Nishigaki, R., and Umemura, K. (1990) Mechanism of Cell Damage by Ultrasound in Combination with Hematoporphyrin. Jpn. J. Cancer Res. 81, 962−966. (79) Lovell, J. F., Liu, T. W. B., Chen, J., and Zheng, G. (2010) Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 110, 2839−2857. (80) Kotkowiak, M., and Dudkowiak, A. (2015) Multiwavelength Excitation of Photosensitizers Interacting with Gold Nanoparticles and Its Impact on Optical Properties of Their Hybrid Mixtures. Phys. Chem. Chem. Phys. 17, 27366−27372. (81) Misík, V. (1996) Recent Applications of Epr and Spin Trapping to Sonochemical Studies of Organic Liquids and Aqueous Solutions. Ultrason. Sonochem. 3, S173−S186. (82) Sazgarnia, A., Shanei, A., Eshghi, H., Hassanzadeh-Khayyat, M., Esmaily, H., and Shanei, M. M. (2013) Detection of Sonoluminescence Signals in a Gel Phantom in the Presence of Protoporphyrin Ix Conjugated to Gold Nanoparticles. Ultrasonics 53, 29−35. (83) Hachimine, K., Shibaguchi, H., Kuroki, M., Yamada, H., Kinugasa, T., Nakae, Y., Asano, R., Sakata, I., Yamashita, Y., Shirakusa, T., et al. (2007) Sonodynamic Therapy of Cancer Using a Novel Porphyrin Derivative, Dcph-P-Na(I), Which Is Devoid of Photosensitivity. Cancer Sci. 98, 916−920. (84) Kessel, D., Jeffers, R., Fowlkes, J. B., and Cain, C. (1994) Porphyrin-Induced Enhancement of Ultrasound Cytotoxicity. Int. J. Radiat. Biol. 66, 221−228. (85) Western, A., Camp, J. R. V., Bensasson, R., Land, E. J., and Kochevar, I. E. (1987) Involvement of Singlet Oxygen in the Phototoxicity Mechanism for a Metabolite of Piroxicam. Photochem. Photobiol. 46, 469−475. (86) Roy, R. A., Ahmad, M., and Crum, L. A. (1994) Physical Mechanisms Governing the Hydrodynamic Response of an Oscillating Ultrasonic File. Int. Endod. J. 27, 197−207. (87) Margulis, M. A. (1995) Sonochemistry and Cavitation, Gordon and Breach Publishers, Australia [Langhorne, Pa., U.S.]. (88) Miller, M. W., Miller, D. L., and Brayman, A. A. (1996) A Review of in Vitro Bioeffects of Inertial Ultrasonic Cavitation from a Mechanistic Perspective. Ultrasound Med. Biol. 22, 1131−1154. (89) Miyoshi, N., Mišík, V., and Riesz, P. (1997) Sonodynamic Toxicity of Gallium-Porphyrin Analogue Atx-70 in Human Leukemia Cells. Radiat. Res. 148, 43−47. (90) Pryor, W. A. (1986) Oxy-Radicals and Related Species: Their Formation, Lifetimes, and Reactions. Annu. Rev. Physiol. 48, 657−667. (91) Moan, J., and Berg, K. (1991) The Photodegradation of Porphyrins in Cells Can Be Used to Estimate the Lifetime of Singlet Oxygen. Photochem. Photobiol. 53, 549−553. (92) Skovsen, E., Snyder, J. W., Lambert, J. D. C., and Ogilby, P. R. (2005) Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chem. B 109, 8570−8573. ́ V. r., and Riesz, P. (2000) Free Radical Intermediates in (93) MišIk, Sonodynamic Therapy. Ann. N. Y. Acad. Sci. 899, 335−348. (94) Armour, E. P., and Corry, P. M. (1982) Cytotoxic Effects of Ultrasound in Vitro Dependence on Gas Content, Frequency, Radical Scavengers, and Attachment. Radiat. Res. 89, 369−380. ́ V. r., Miyoshi, N., and Riesz, P. (1999) Effects of (95) MišIk, Cysteamine and Cystamine on the Sonochemical Accumulation of Hydrogen PeroxideImplications for Their Mechanisms of Action in Ultrasound-Exposed Cells. Free Radical Biol. Med. 26, 961−967. (96) Church, C. C., and Miller, M. W. (1983) The Kinetics and Mechanics of Ultrasonically-Induced Cell Lysis Produced by NonTrapped Bubbles in a Rotating Culture Tube. Ultrasound Med. Biol. 9, 385−393. (97) Inoue, M., Church, C. C., Brayman, A., Miller, M. W., and Malcuit, M. S. (1989) Confirmation of the Protective Effect of Cysteamine in in Vitro Ultrasound Exposures. Ultrasonics 27, 362− 369. Review (98) Secomski, W., Bilmin, K., Kujawska, T., Nowicki, A., Grieb, P., and Lewin, P. A. (2017) In Vitro Ultrasound Experiments: Standing Wave and Multiple Reflections Influence on the Outcome. Ultrasonics 77, 203−213. (99) Nyborg, W. L., and Miller, D. L. (1983) Physical Mechanisms for Biological Effects of Ultrasound at Low-Intensity Levels. In Ultrasound Interactions in Biology and Medicine (Millner, R., Rosenfeld, E., and Cobet, U., Eds.) pp 131−138, Springer US, Boston. (100) Miller, M. W., Miller, D. L., and Brayman, A. A. (1996) A Review of in Vitro Bioeffects of Inertial Ultrasonic Cavitation from a Mechanistic Perspective. Ultrasound Med. Biol. 22, 1131−1154. (101) Clarke, P. R., and Hill, C. R. (1970) Physical and Chemical Aspects of Ultrasonic Disruption of Cells. J. Acoust. Soc. Am. 47, 649− 653. (102) Dunn, F. (1985) Cellular Inactivation by Heat and Shear. Radiat. Environ. Biophys. 24, 131−139. (103) Worthington, A. E., Thompson, J., Lalonde, R., Patterson, M., Rauth, A. M., and Hunt, J. W. (1997) Mechanism of Ultrasound Enhanced Porphyrin Cytotoxicity: Free Radical and Hematoporphryn Effects. IEEE Ultrason. Symp. 2, 1349−1352. (104) Worthington, A. E., Thompson, J., Rauth, A. M., and Hunt, J. W. (1997) Mechanism of Ultrasound Enhanced Porphyrin Cytotoxicity. Part I: A Search for Free Radical Effects. Ultrasound Med. Biol. 23, 1095−1105. (105) Reitman, L. W., Char, D. H., and Bernstein, E. F. (1970) Effect of A-Tocopherol on Lipid Peroxide Production and Hemolysis Following Mechanical Trauma to Blood. J. Surg. Res. 10, 471−476. (106) Miller, M. W., Miller, W. M., and Battaglia, L. F. (2003) Biological and Environmental Factors Affecting Ultrasound-Induced Hemolysis in Vitro: 3. Antioxidant (Trolox®) Inclusion. Ultrasound Med. Biol. 29, 103−112. (107) Brault, D. (1990) Physical Chemistry of Porphyrins and Their Interactions with Membranes: The Importance of pH. J. Photochem. Photobiol., B 6, 79−86. (108) Feril, L. B., Kondo, T., Cui, Z.-G., Tabuchi, Y., Zhao, Q.-L., Ando, H., Misaki, T., Yoshikawa, H., and Umemura, S.-i. (2005) Apoptosis Induced by the Sonomechanical Effects of Low Intensity Pulsed Ultrasound in a Human Leukemia Cell Line. Cancer Lett. 221, 145−152. (109) McEwan, C., Owen, J., Stride, E., Fowley, C., Nesbitt, H., Cochrane, D., Coussios, C. C., Borden, M., Nomikou, N., McHale, A. P., et al. (2015) Oxygen Carrying Microbubbles for Enhanced Sonodynamic Therapy of Hypoxic Tumours. J. Controlled Release 203, 51−56. (110) Costley, D., Mc Ewan, C., Fowley, C., McHale, A. P., Atchison, J., Nomikou, N., and Callan, J. F. (2015) Treating Cancer with Sonodynamic Therapy: A Review. Int. J. Hyperthermia 31, 107− 117. (111) Wan, G. Y., Liu, Y., Chen, B. W., Liu, Y. Y., Wang, Y. S., Zhang, N., et al. (2016) Recent Advances of Sonodynamic Therapy in Cancer Treatment. Cancer Biol. Med. 13, 325−338. (112) Lafond, M., Yoshizawa, S., and Umemura, S. I. (2019) Sonodynamic Therapy: Advances and Challenges in Clinical Translation. J. Ultrasound Med. 38, 567−580. (113) Rosenthal, I., Sostaric, J. Z., and Riesz, P. (2004) Sonodynamic Therapy - a Review of the Synergistic Effects of Drugs and Ultrasound. Ultrason. Sonochem. 11, 349−363. (114) Maeda, M., Muragaki, Y., Okamoto, J., Yoshizawa, S., Abe, N., Nakamoto, H., Ishii, H., Kawabata, K., Umemura, S., Nishiyama, N., et al. (2017) Sonodynamic Therapy Based on Combined Use of Low Dose Administration of Epirubicin-Incorporating Drug Delivery System and Focused Ultrasound. Ultrasound Med. Biol. 43, 2295− 2301. (115) Li, Y., Zhou, Q., Deng, Z., Pan, M., Liu, X., Wu, J., Yan, F., and Zheng, H. (2016) Ir-780 Dye as a Sonosensitizer for Sonodynamic Therapy of Breast Tumor. Sci. Rep. 6, 25968. (116) Goldberg, B. B., Liu, J.-B., and Forsberg, F. (1994) Ultrasound Contrast Agents: A Review. Ultrasound Med. Biol. 20, 319−333. T https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc (117) Hernot, S., and Klibanov, A. L. (2008) Microbubbles in Ultrasound-Triggered Drug and Gene Delivery. Adv. Drug Delivery Rev. 60, 1153−1166. (118) Unger, E. C., Hersh, E., Vannan, M., Matsunaga, T. O., and McCreery, T. (2001) Local Drug and Gene Delivery through Microbubbles. Prog. Cardiovasc. Dis. 44, 45−54. (119) Nomikou, N., Fowley, C., Byrne, N. M., McCaughan, B., McHale, A. P., and Callan, J. F. (2012) Microbubble-Sonosensitiser Conjugates as Therapeutics in Sonodynamic Therapy. Chem. Commun. 48, 8332−8334. (120) Beguin, E., Shrivastava, S., Dezhkunov, N. V., McHale, A. P., Callan, J. F., and Stride, E. (2019) Direct Evidence of Multibubble Sonoluminescence Using Therapeutic Ultrasound and Microbubbles. ACS Appl. Mater. Interfaces 11, 19913−19919. (121) Logan, K., Foglietta, F., Nesbitt, H., Sheng, Y., McKaig, T., Kamila, S., Gao, J., Nomikou, N., Callan, B., McHale, A. P., et al. (2019) Targeted Chemo-Sonodynamic Therapy Treatment of Breast Tumours Using Ultrasound Responsive Microbubbles Loaded with Paclitaxel, Doxorubicin and Rose Bengal. Eur. J. Pharm. Biopharm. 139, 224−231. (122) Nesbitt, H., Sheng, Y., Kamila, S., Logan, K., Thomas, K., Callan, B., Taylor, M. A., Love, M., O’Rourke, D., Kelly, P., et al. (2018) Gemcitabine Loaded Microbubbles for Targeted ChemoSonodynamic Therapy of Pancreatic Cancer. J. Controlled Release 279, 8−16. (123) McEwan, C., Owen, J., Stride, E., Fowley, C., Nesbitt, H., Cochrane, D., Coussios, C. C., Borden, M., Nomikou, N., McHale, A. P., et al. (2015) Oxygen Carrying Microbubbles for Enhanced Sonodynamic Therapy of Hypoxic Tumours. J. Controlled Release 203, 51−56. (124) Singh, R., and Lillard, J. W., Jr. (2009) Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 86, 215−223. (125) Ahmed, S. E., Martins, A. M., and Husseini, G. A. (2015) The Use of Ultrasound to Release Chemotherapeutic Drugs from Micelles and Liposomes. J. Drug Target. 23, 16−42. (126) Rajora, M. A., Lou, J. W. H., and Zheng, G. (2017) Advancing Porphyrin’s Biomedical Utility Via Supramolecular Chemistry. Chem. Soc. Rev. 46, 6433−6469. (127) Huang, P., Qian, X., Chen, Y., Yu, L., Lin, H., Wang, L., Zhu, Y., and Shi, J. (2017) Metalloporphyrin-Encapsulated Biodegradable Nanosystems for Highly Efficient Magnetic Resonance ImagingGuided Sonodynamic Cancer Therapy. J. Am. Chem. Soc. 139, 1275− 1284. (128) Liu, Y., Bai, L., Guo, K., Jia, Y., Zhang, K., Liu, Q., Wang, P., and Wang, X. (2019) Focused Ultrasound-Augmented Targeting Delivery of Nanosonosensitizers from Homogenous Exosomes for Enhanced Sonodynamic Cancer Therapy. Theranostics 9, 5261−5281. (129) Zhang, Y., Ou, Y., Guo, J., and Huang, X. (2020) UltrasoundTriggered Breast Tumor Sonodynamic Therapy through Hematoporphyrin Monomethyl Ether-Loaded Liposome. J. Biomed. Mater. Res., Part B 108, 948. (130) Wang, X., Yan, F., Liu, X., Wang, P., Shao, S., Sun, Y., Sheng, Z., Liu, Q., Lovell, J. F., and Zheng, H. (2018) Enhanced Drug Delivery Using Sonoactivatable Liposomes with Membrane-Embedded Porphyrins. J. Controlled Release 286, 358−368. (131) Harada, A., Ono, M., Yuba, E., and Kono, K. (2013) Titanium Dioxide Nanoparticle-Entrapped Polyion Complex Micelles Generate Singlet Oxygen in the Cells by Ultrasound Irradiation for Sonodynamic Therapy. Biomater. Sci. 1, 65−73. (132) Deepagan, V. G., You, D. G., Um, W., Ko, H., Kwon, S., Choi, K. Y., Yi, G.-R., Lee, J. Y., Lee, D. S., Kim, K., et al. (2016) LongCirculating Au-Tio2 Nanocomposite as a Sonosensitizer for RosMediated Eradication of Cancer. Nano Lett. 16, 6257−6264. (133) Dai, C., Zhang, S., Liu, Z., Wu, R., and Chen, Y. (2017) TwoDimensional Graphene Augments Nanosonosensitized Sonocatalytic Tumor Eradication. ACS Nano 11, 9467−9480. (134) Yamaguchi, S., Kobayashi, H., Narita, T., Kanehira, K., Sonezaki, S., Kudo, N., Kubota, Y., Terasaka, S., and Houkin, K. (2011) Sonodynamic Therapy Using Water-Dispersed Tio2-Poly- Review ethylene Glycol Compound on Glioma Cells: Comparison of Cytotoxic Mechanism with Photodynamic Therapy. Ultrason. Sonochem. 18, 1197−1204. (135) Harada, Y., Ogawa, K., Irie, Y., Endo, H., Feril, L. B., Uemura, T., and Tachibana, K. (2011) Ultrasound Activation of Tio2 in Melanoma Tumors. J. Controlled Release 149, 190−195. (136) Moosavi Nejad, S., Takahashi, H., Hosseini, H., Watanabe, A., Endo, H., Narihira, K., Kikuta, T., and Tachibana, K. (2016) Acute Effects of Sono-Activated Photocatalytic Titanium Dioxide Nanoparticles on Oral Squamous Cell Carcinoma. Ultrason. Sonochem. 32, 95−101. (137) Ninomiya, K., Ogino, C., Oshima, S., Sonoke, S., Kuroda, S.-i., and Shimizu, N. (2012) Targeted Sonodynamic Therapy Using Protein-Modified Tio2 Nanoparticles. Ultrason. Sonochem. 19, 607− 614. (138) Ninomiya, K., Fukuda, A., Ogino, C., and Shimizu, N. (2014) Targeted Sonocatalytic Cancer Cell Injury Using Avidin-Conjugated Titanium Dioxide Nanoparticles. Ultrason. Sonochem. 21, 1624−1628. (139) You, D. G., Deepagan, V. G., Um, W., Jeon, S., Son, S., Chang, H., Yoon, H. I., Cho, Y. W., Swierczewska, M., Lee, S., et al. (2016) Ros-Generating Tio2 Nanoparticles for Non-Invasive Sonodynamic Therapy of Cancer. Sci. Rep. 6, 23200. (140) Feng, Q., Yang, X., Hao, Y., Wang, N., Feng, X., Hou, L., and Zhang, Z. (2019) Cancer Cell Membrane-Biomimetic Nanoplatform for Enhanced Sonodynamic Therapy on Breast Cancer Via Autophagy Regulation Strategy. ACS Appl. Mater. Interfaces 11, 32729−32738. (141) Kim, S., Im, S., Park, E.-Y., Lee, J., Kim, C., Kim, T.-i., and Kim, W. J. (2020) Drug-Loaded Titanium Dioxide Nanoparticle Coated with Tumor Targeting Polymer as a Sonodynamic Chemotherapeutic Agent for Anti-Cancer Therapy. Nanomedicine 24, 102110. (142) Tuziuti, T., Yasui, K., Sivakumar, M., Iida, Y., and Miyoshi, N. (2005) Correlation between Acoustic Cavitation Noise and Yield Enhancement of Sonochemical Reaction by Particle Addition. J. Phys. Chem. A 109, 4869−4872. (143) Pan, X., Bai, L., Wang, H., Wu, Q., Wang, H., Liu, S., Xu, B., Shi, X., and Liu, H. (2018) Metal-Organic-Framework-Derived Carbon Nanostructure Augmented Sonodynamic Cancer Therapy. Adv. Mater. 30, No. e1800180. (144) Chen, J., Luo, H., Liu, Y., Zhang, W., Li, H., Luo, T., Zhang, K., Zhao, Y., and Liu, J. (2017) Oxygen-Self-Produced Nanoplatform for Relieving Hypoxia and Breaking Resistance to Sonodynamic Treatment of Pancreatic Cancer. ACS Nano 11, 12849−12862. (145) Wang, Y., Liu, Y., Wu, H., Zhang, J., Tian, Q., and Yang, S. (2019) Functionalized Holmium-Doped Hollow Silica Nanospheres for Combined Sonodynamic and Hypoxia-Activated Therapy. Adv. Funct. Mater. 29, 1805764. (146) Yue, W., Chen, L., Yu, L., Zhou, B., Yin, H., Ren, W., Liu, C., Guo, L., Zhang, Y., Sun, L., et al. (2019) Checkpoint Blockade and Nanosonosensitizer-Augmented Noninvasive Sonodynamic Therapy Combination Reduces Tumour Growth and Metastases in Mice. Nat. Commun. 10, 2025. (147) Shimizu, N., Ninomiya, K., Ogino, C., and Rahman, M. M. (2010) Potential Uses of Titanium Dioxide in Conjunction with Ultrasound for Improved Disinfection. Biochem. Eng. J. 48, 416−423. (148) Brazzale, C., Canaparo, R., Racca, L., Foglietta, F., Durando, G., Fantozzi, R., Caliceti, P., Salmaso, S., and Serpe, L. (2016) Enhanced Selective Sonosensitizing Efficacy of Ultrasound-Based Anticancer Treatment by Targeted Gold Nanoparticles. Nanomedicine 11, 3053−3070. (149) Gorgizadeh, M., Azarpira, N., Lotfi, M., Daneshvar, F., Salehi, F., and Sattarahmady, N. (2019) Sonodynamic Cancer Therapy by a Nickel Ferrite/Carbon Nanocomposite on Melanoma Tumor: In Vitro and in Vivo Studies. Photodiagn. Photodyn. Ther. 27, 27−33. (150) Chen, Y.-W., Liu, T.-Y., Chang, P.-H., Hsu, P.-H., Liu, H.-L., Lin, H.-C., and Chen, S.-Y. (2016) A Theranostic Nrgo@Msn-Ion Nanocarrier Developed to Enhance the Combination Effect of Sonodynamic Therapy and Ultrasound Hyperthermia for Treating Tumor. Nanoscale 8, 12648−12657. U https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review on-Demand Boosted Drug Release During Sdt Process. Adv. Healthcare Mater. 8, No. e1900720. (168) Liu, M., Khan, A. R., Ji, J., Lin, G., Zhao, X., and Zhai, G. (2018) Crosslinked Self-Assembled Nanoparticles for ChemoSonodynamic Combination Therapy Favoring Antitumor, Antimetastasis Management and Immune Responses. J. Controlled Release 290, 150−164. (169) Malekmohammadi, S., Hadadzadeh, H., Rezakhani, S., and Amirghofran, Z. (2019) Design and Synthesis of Gatekeeper Coated Dendritic Silica/Titania Mesoporous Nanoparticles with Sustained and Controlled Drug Release Properties for Targeted Synergetic Chemo-Sonodynamic Therapy. ACS Biomater. Sci. Eng. 5, 4405− 4415. (170) Wu, P., Sun, Y., Dong, W., Zhou, H., Guo, S., Zhang, L., Wang, X., Wan, M., and Zong, Y. (2019) Enhanced Anti-Tumor Efficacy of Hyaluronic Acid Modified Nanocomposites Combined with Sonochemotherapy against Subcutaneous and Metastatic Breast Tumors. Nanoscale 11, 11470−11483. (171) Shanei, A., and Akbari-Zadeh, H. (2019) Investigating the Sonodynamic-Radiosensitivity Effect of Gold Nanoparticles on Hela Cervical Cancer Cells. J. Korean Med. Sci. 34, No. e243. (172) Liang, S., Deng, X., Chang, Y., Sun, C., Shao, S., Xie, Z., Xiao, X., Ma, P. a., Zhang, H., Cheng, Z., et al. (2019) Intelligent Hollow Pt-Cus Janus Architecture for Synergistic Catalysis-Enhanced Sonodynamic and Photothermal Cancer Therapy. Nano Lett. 19, 4134−4145. (173) Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T. D., Mazur, M., and Telser, J. (2007) Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 39, 44−84. (174) Gamcsik, M. P., Kasibhatla, M. S., Teeter, S. D., and Colvin, O. M. (2012) Glutathione Levels in Human Tumors. Biomarkers 17, 671−691. (175) Gong, F., Cheng, L., Yang, N., Betzer, O., Feng, L., Zhou, Q., Li, Y., Chen, R., Popovtzer, R., and Liu, Z. (2019) Ultrasmall OxygenDeficient Bimetallic Oxide Mnwox Nanoparticles for Depletion of Endogenous Gsh and Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 31, 1900730. (176) Wang, L., Niu, M., Zheng, C., Zhao, H., Niu, X., Li, L., Hu, Y., Zhang, Y., Shi, J., and Zhang, Z. (2018) A Core-Shell Nanoplatform for Synergistic Enhanced Sonodynamic Therapy of Hypoxic Tumor Via Cascaded Strategy. Adv. Healthcare Mater. 7, 1800819. (177) Zhang, H., Zhang, X., Ren, Y., Cao, F., Hou, L., and Zhang, Z. (2019) An in Situ Microenvironmental Nano-Regulator to Inhibit the Proliferation and Metastasis of 4T1 Tumor. Theranostics 9, 3580− 3594. (178) Xie, L., Feng, X., Huang, M., Zhang, K., and Liu, Q. (2019) Sonodynamic Therapy Combined to 2-Deoxyglucose Potentiate Cell Metastasis Inhibition of Breast Cancer. Ultrasound Med. Biol. 45, 2984−2992. (179) Zhang, Q., Bao, C., Cai, X., Jin, L., Sun, L., Lang, Y., and Li, L. (2018) Sonodynamic Therapy-Assisted Immunotherapy: A Novel Modality for Cancer Treatment. Cancer Sci. 109, 1330−1345. (180) Kenyon, J. N., Fulle, R. J., and Lewis, T. J. (2009) Activated Cancer Therapy Using Light and Ultrasound - a Case Series of Sonodynamic Photodynamic Therapy in 115 Patients over a 4 Year Period. Curr. Drug Ther. 4, 179−193. (181) McCaughan, B., Rouanet, C., Fowley, C., Nomikou, N., McHale, A. P., McCarron, P. A., and Callan, J. F. (2011) Enhanced Ros Production and Cell Death through Combined Photo- and SonoActivation of Conventional Photosensitising Drugs. Bioorg. Med. Chem. Lett. 21, 5750−5752. (182) Jun-Qun, Z., Guo-Min, W., Bo, Y., Gong-Xian, W., and ShenXu, H. (2004) Short-Term Results of 89 Cases of Rectal Carcinoma Treated with High-Intensity Focused Ultrasound and Low-Dose Radiotherapy. Ultrasound Med. Biol. 30, 57−60. (183) Beguin, E., Gray, M. D., Logan, K. A., Nesbitt, H., Sheng, Y., Kamila, S., Barnsley, L. C., Bau, L., McHale, A. P., Callan, J. F., et al. (2020) Magnetic Microbubble Mediated Chemo-Sonodynamic (151) Osminkina, L. A., Nikolaev, A. L., Sviridov, A. P., Andronova, N. V., Tamarov, K. P., Gongalsky, M. B., Kudryavtsev, A. A., Treshalina, H. M., and Timoshenko, V. Y. (2015) Porous Silicon Nanoparticles as Efficient Sensitizers for Sonodynamic Therapy of Cancer. Microporous Mesoporous Mater. 210, 169−175. (152) Zhong, X., Wang, X., Cheng, L., Tang, Y. a., Zhan, G., Gong, F., Zhang, R., Hu, J., Liu, Z., and Yang, X. (2020) Gsh-Depleted Ptcu3 Nanocages for Chemodynamic- Enhanced Sonodynamic Cancer Therapy. Adv. Funct. Mater. 30, 1907954. (153) Zhao, Y., Zhu, Y., Fu, J., and Wang, L. (2014) Effective Cancer Cell Killing by Hydrophobic Nanovoid-Enhanced Cavitation under Safe Low-Energy Ultrasound. Chem. - Asian J. 9, 790−796. (154) Kwan, J. J., Myers, R., Coviello, C. M., Graham, S. M., Shah, A. R., Stride, E., Carlisle, R. C., and Coussios, C. C. (2015) UltrasoundPropelled Nanocups for Drug Delivery. Small 11, 5305−5314. (155) Bhatnagar, S., Kwan, J. J., Shah, A. R., Coussios, C.-C., and Carlisle, R. C. (2016) Exploitation of Sub-Micron Cavitation Nuclei to Enhance Ultrasound-Mediated Transdermal Transport and Penetration of Vaccines. J. Controlled Release 238, 22−30. (156) Zhang, H., Chen, J., Zhu, X., Ren, Y., Cao, F., Zhu, L., Hou, L., Zhang, H., and Zhang, Z. (2018) Ultrasound Induced PhaseTransition and Invisible Nanobomb for Imaging-Guided Tumor Sonodynamic Therapy. J. Mater. Chem. B 6, 6108−6121. (157) Feng, Q., Li, Y., Yang, X., Zhang, W., Hao, Y., Zhang, H., Hou, L., and Zhang, Z. (2018) Hypoxia-Specific Therapeutic Agents Delivery Nanotheranostics: A Sequential Strategy for Ultrasound Mediated on-Demand Tritherapies and Imaging of Cancer. J. Controlled Release 275, 192−200. (158) Bosca, F., Bielecki, P. A., Exner, A. A., and Barge, A. (2018) Porphyrin-Loaded Pluronic Nanobubbles: A New Us-Activated Agent for Future Theranostic Applications. Bioconjugate Chem. 29, 234−240. (159) Chang, N., Qin, D., Wu, P., Xu, S., Wang, S., and Wan, M. (2019) Ir780 Loaded Perfluorohexane Nanodroplets for Efficient Sonodynamic Effect Induced by Short-Pulsed Focused Ultrasound. Ultrason. Sonochem. 53, 59−67. (160) Zhang, L., Yi, H., Song, J., Huang, J., Yang, K., Tan, B., Wang, D., Yang, N., Wang, Z., and Li, X. (2019) Mitochondria-Targeted and Ultrasound-Activated Nanodroplets for Enhanced Deep-Penetration Sonodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 11, 9355− 9366. (161) Jin, Q., Kang, S.-T., Chang, Y.-C., Zheng, H., and Yeh, C.-K. (2016) Inertial Cavitation Initiated by Polytetrafluoroethylene Nanoparticles under Pulsed Ultrasound Stimulation. Ultrason. Sonochem. 32, 1−7. (162) Zhu, J., Chu, C., Li, D., Pang, X., Zheng, H., Wang, J., Shi, Y., Zhang, Y., Cheng, Y., Ren, E., et al. (2019) Fe(Iii)-Porphyrin Sonotheranostics: A Green Triple-Regulated Ros Generation Nanoplatform for Enhanced Cancer Imaging and Therapy. Adv. Funct. Mater. 29, 1904056. (163) Zhou, C., Xie, X., Yang, H., Zhang, S., Li, Y., Kuang, C., Fu, S., Cui, L., Liang, M., Gao, C., et al. (2019) Novel Class of UltrasoundTriggerable Drug Delivery Systems for the Improved Treatment of Tumors. Mol. Pharmaceutics 16, 2956−2965. (164) Bakhshizadeh, M., Moshirian, T., Esmaily, H., Rajabi, O., Nassirli, H., and Sazgarnia, A. (2017) Sonophotodynamic Therapy Mediated by Liposomal Zinc Phthalocyanine in a Colon Carcinoma Tumor Model: Role of Irradiating Arrangement. Iran J. Basic Med. Sci. 20, 1088−1092. (165) Han, X., Huang, J., Jing, X., Yang, D., Lin, H., Wang, Z., Li, P., and Chen, Y. (2018) Oxygen Deficient Black Titania for Synergistic/ Enhanced Sonodynamic and Photoinduced Cancer Therapy at Near Infrared-II Biowindow. ACS Nano 12, 4545−4555. (166) Gao, F., He, G., Yin, H., Chen, J., Liu, Y., Lan, C., Zhang, S., and Yang, B. (2019) Titania-Coated 2d Gold Nanoplates as Nanoagents for Synergistic Photothermal/Sonodynamic Therapy in the Second near-Infrared Window. Nanoscale 11, 2374−2384. (167) Wu, P., Dong, W., Guo, X., Qiao, X., Guo, S., Zhang, L., Wan, M., and Zong, Y. (2019) Ros-Responsive Blended Nanoparticles: Cascade-Amplifying Synergistic Effects of Sonochemotherapy with V https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX Bioconjugate Chemistry pubs.acs.org/bc Review Therapy Using a Combined Magnetic-Acoustic Device. J. Controlled Release 317, 23−33. (184) Jones, R. M., and Hynynen, K. (2019) Advances in Acoustic Monitoring and Control of Focused Ultrasound-Mediated Increases in Blood-Brain Barrier Permeability. Br. J. Radiol. 92, 20190672. (185) Blanco, E., Shen, H., and Ferrari, M. (2015) Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 33, 941−951. (186) Hoshyar, N., Gray, S., Han, H., and Bao, G. (2016) The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 11, 673−692. (187) Pellow, C., Goertz, D. E., and Zheng, G. (2018) Breaking Free from Vascular Confinement: Status and Prospects for Submicron Ultrasound Contrast Agents. WIREs Nanomed. Nanobi. 10, No. e1502. (188) Huang, S.-L., and MacDonald, R. C. (2004) Acoustically Active Liposomes for Drug Encapsulation and Ultrasound-Triggered Release. Biochim. Biophys. Acta, Biomembr. 1665, 134−141. (189) Huynh, E., Leung, B. Y. C., Helfield, B. L., Shakiba, M., Gandier, J.-A., Jin, C. S., Master, E. R., Wilson, B. C., Goertz, D. E., and Zheng, G. (2015) In Situ Conversion of Porphyrin Microbubbles to Nanoparticles for Multimodality Imaging. Nat. Nanotechnol. 10, 325. (190) Wang, X., Zhang, W., Xu, Z., Luo, Y., Mitchell, D., and Moss, R. W. (2009) Sonodynamic and Photodynamic Therapy in Advanced Breast Carcinoma: A Report of 3 Cases. Integr. Cancer Ther. 8, 283− 287. (191) Inui, T., Makita, K., Miura, H., Matsuda, A., Kuchiike, D., Kubo, K., Mette, M., Uto, Y., Nishikata, T., Hori, H., et al. (2014) Case Report: A Breast Cancer Patient Treated with Gcmaf, Sonodynamic Therapy and Hormone Therapy. Anticancer Res. 34, 4589−4593. (192) Bachurin, S., Bukatina, E., Lermontova, N., Tkachenko, S., Afanasiev, A., Grigoriev, V., Grigorieva, I., Ivanov, Y. U., Sablin, S., and Zefirov, N. (2001) Antihistamine Agent Dimebon as a Novel Neuroprotector and a Cognition Enhancer. Ann. N. Y. Acad. Sci. 939, 425−435. (193) Steele, J. W., Kim, S. H., Cirrito, J. R., Verges, D. K., Restivo, J. L., Westaway, D., Fraser, P., Hyslop, P. S. G., Sano, M., Bezprozvanny, I., et al. (2009) Acute Dosing of Latrepirdine (Dimebon), a Possible Alzheimer Therapeutic, Elevates Extracellular Amyloid-B Levels in Vitro and in Vivo. Mol. Neurodegener. 4, 51. W https://dx.doi.org/10.1021/acs.bioconjchem.0c00029 Bioconjugate Chem. XXXX, XXX, XXX−XXX