Chemistry Test: Atomic Structure, Molarity, and Molecular Mass
advertisement

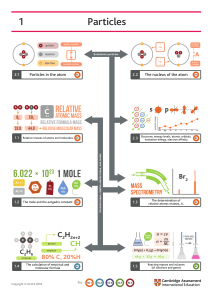
(1) Chooce the correct answer :× Atomic number of dutarium is – (a)1 (b) 3 (c)3 (d) 6 (2) Molarity of pure water is-18 (a)18 (b)50 (c)55.6 (d)100 (3) The ratio of wasses of Qxygen and Nitrogen in particular gases mixture is 1:4 the ratio of the number of their Molucular is _ (a) 3:16 (b) 1:4 (c) 7:32 (d) 1:8 (4) The number of water molecule is maximum in (a) 18 gram of water (b) 18 moles of water (c) 18 molucules of water (d) 1.8 gram of water (5) Idefiy use pair which are not a isotopes(a)12×6,13y6 (b)35×17,37×17 (c)14×6,14×7 (d)8×4,8y5 (2) Fill in the blank : 1. …………………. Is known as father of chemistry. 2. Empirical formula of acetic acid is ……………… . 3. Nahco2 +ch5 cooh ………………….+……………… . 4 ………………. Is einsteins eqution . 5 Substances which take part in reactions are called …………………….. . (2) Macth the following (1) Law of mass conservation (2) Molecular formula (3) Photon (4) Electron (5) Azimutual quantum number - Exhibitsboth momentary Shape of orbital Alwas have negative sign Lavoisier Emiprical formula X n 4. Short answers: 1 define a molecular mass with example. 2 define a atomic mass and atomic number. 3 Calculate the total number of electrons present. In one mole of methane=6.022× 102 4 4. Explain mole fraction. 5. define wavelenghtand velocity. 5.Very short answers. a. Fiving and Electrons in orbitals b.Electronic configration of elements 2×5=10 1S 2 CL 3 NE 4 AL 5F Long answer: 1 calculate the molecular mass of the following (a)H2O (b)CO2