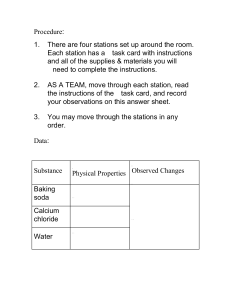
UNKNOWN LAB Name: ________________________________ SAFETY INFORMATION● DO NOT EAT THE IODINE. **Iodine is in group 17! It’s a Halogen - and is super reactive. DO NOT CONSUME. ○ You need small amounts in your body (if your body doesn’t absorb enough iodine, you can end up with a goiter… but most salt sold in the USA is iodized, so you are most likely getting more than enough). ● Wash your hands as soon as you finish with the lab! You don’t want the iodine on your hands. ● liquid iodine will stain. Be careful. ____________________________________________________________________________________________________________________________________ Background Information: When 2 or more substances are brought together, 1 of 3 things happens: 1. They react (or undergo a chemical change). This happens because bonds between atoms in the ___________________ (starting materials) break, and new bonds form. At the end of a chemical change, you are left with ____________________ that have new properties than the original substance. a. Chemical changes happen because both reactants have the chemical property of reactivity with each other 2. One substance is dissolved in the other, forming a mixture called a solution. This is a physical change. a. Solutions are formed when the starting materials have the physical property of solubility. b. The starting materials do NOT have reactivity with each other c. FYI, in this case, the thing being dissolved is called the solute and the thing doing the dissolving in the solvent 3. The materials stay separate. In this case, the substances don’t have the chemical property of reactivity, or the physical property of solubility. a. An example of this would be attempting to mix oil and water. They are incompatible and neither mix nor react Data Table PROPERTIES Baking Powder Obs type Appearance Texture Baking Soda Density Appearance Texture Cornstarch or Flour Density Appearance Texture Sugar Density Appearance Texture Density Change Type Property Observations Data Table CHANGES Baking Powder Water Baking Soda Observation Change Type Property bubbles chemical Reactivity with water Observation Change Type Cornstarch or Flour Property Observation Change Type Sugar Property Observation Iodine Vinegar Data Table UNKNOWN Observations of your unknown? Unknown _________ Observation Water Iodine Vinegar Change Type Unknown ___________ Property Observation Change Type Unknown ___________ Property Observation Change Type Unknown ___________ Property Observation Change Type Property UNKNOWN LAB PROCEDURE: 1. Look at each substance & make observations in the “properties” table above. 2. Using a graduated cylinder & large pipet, carefully measure out 2.5ml of water from the plastic beaker and place into 5 test tubes. 3. Fold a small paper in half and zero it onto the balance. Measure out ~0.1g of baking powder. Place into the first test tube. “Shake” the test tube using the thumping method. Observe (with ears & eyes!) what happens. Record. 4. Repeat step 2 with ~0.2g of baking soda in the 2nd test tube. Observe & record. 5. Repeat step 2 with ~0.2g of cornstarch in the 3rd test tube. Observe & record. 6. Repeat step 2 with ~0.2g of sugar in the 4th test tube. Observe & record. 7. Keep the last test tube empty. 8. Add 2 drops of iodine to test tubes 1-5. Thump the test tube to mix. Compare each mixture/reaction to the 5th test tube. Record in the data table. 9. Send 1 person in your group to rinse each test tube. Everything may go down the sink. 10. Using a small pipet & vinegar, carefully add 1 pipet full of vinegar to 5 test tubes. 11. Fold a small paper in half and zero it onto the balance. Measure out ~0.1g of baking powder. Place into the first test tube. “Shake” the test tube using the thumping method. Observe (with ears & eyes!) what happens. Record. 12. Repeat step 2 with ~0.2g of baking soda in the 2nd test tube. Observe & record. 13. Repeat step 2 with ~0.2g of cornstarch in the 3rd test tube. Observe & record. 14. Repeat step 2 with ~0.2g of sugar in the 4th test tube. Observe & record. 15. Keep the last test tube empty.