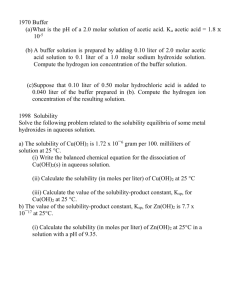
Homework 3 Chemical equilibria Part 1 Aqueous Solutions and Chemical Equilibria 1. Briefly describe or define and give an example of (a) a weak electrolyte. (b) a Brønsted-Lowry acid. (c) the conjugate acid of a Brønsted-Lowry base. (d) neutralization, in terms of the Brønsted-Lowry concept. (e) an amphiprotic solvent. (f) autoprotolysis. (g) a strong acid. (i) the common-ion effect. 2. Briefly describe or define and give an example of (a) an amphiprotic solute. (b) a differentiating solvent. (c) a leveling solvent. 3. Identify the acid and base on the left and its conjugate base and conjugate acid on the right in the following equations: 4. Write expressions for the autoprotolysis of (a) H2O. (b) CH3COOH. (c) CH3NH2. (d) CH3OH. 5. Write the equilibrium-constant expressions and obtain numerical values for each constant in (a) the basic dissociation of aniline, C6H5NH2. (b) the acidic dissociation of hypochlorous acid, HClO. (c) the acidic dissociation of methyl ammonium hydrochloride, CH3NH3Cl. (d) the basic dissociation of NaNO2. 6. Generate the solubility-product expression for (a) CuBr, (b) HgClI and (c) PbCl2. 7. Calculate the solubility-product constant for each of the following substances, given that the molar concentrations of their saturated solutions are as indicated: (a) RaSO4 (6.6 x 10-6 M). (b) Ce(IO3)3 (1.9 x 10-3 M). 8. What CrO42- concentration is required to (a) initiate precipitation of Ag2CrO4 from a solution that is 4.13 x 10-3 M in Ag+? (b) lower the concentration of Ag+ in a solution to 9.00 x 10-7 M? 9. The solubility-product constant for Ce(IO3)3 is 3.2 x 10-10. What is the Ce3+ concentration in a solution prepared by mixing 50.00 mL of 0.0450 M Ce3+ with 50.00 mL of (a) water and (b) 0.0450 M IO3- ? 10. The solubility products for a series of iodides are List these four compounds in order of decreasing molar solubility in (a) water and (b) 0.20 M NaI. 11. Calculate the pH of water at 25°C and 75°C. The values for pKw at these temperatures are 13.99 and 12.70, respectively. 12. At 25°C, what are the molar H3O+ and OH- concentrations in 0.0300 M C6H5COOH? For benzoic acid, Ka = 6.28 × 10–5. 13. At 25°C, what is the hydronium ion concentration in (a) 0.200 M chloroacetic acid and (b) 0.200 M sodium chloroacetate? 14. Calculate pH of the solution prepared by dissolving 8.00 mmol of sodium acetate in 200 mL of 0.100 M acetic acid. 15. What mass of sodium formate (HCOONa) must be added to 500.0 mL of 1.00 M formic acid to produce a buffer solution that has a pH of 3.50? 16. What volume of 0.200 M HCl must be added to 500.0 mL of 0.300 M sodium mandelate to produce a buffer solution with a pH of 3.37? Part 2 Effect of Electrolytes on Chemical Equilibria 1. Neglecting any effects caused by volume changes, would you expect the ionic strength to (1) increase, (2) decrease, or (3) remain essentially unchanged when NaOH is added to a dilute solution of (a) magnesium chloride [Mg(OH)2(s) forms]? (b) hydrochloric acid? (c) acetic acid? 2. Calculate the ionic strength of a solution that is (a) 0.030 M in FeSO4. (b) 0.30 M in FeCl3 and 0.20 M in FeCl2. 3. Calculate the activity coefficient of (a) Fe3+ at = 0.062 and (b) Ce4+ at = 0.070. X of Fe3+: 0.9, X of Ce4+: 1.1 4. For a solution in which = 8.0 x 10-2, calculate K’sp for AgSCN. For Ag+, Ag+ = 0.25. For SCN–, SCN– = 0.35. 5. Use activities to calculate the molar solubility of Zn(OH)2 in (a) 0.0200 M KCl and (b) 0.0300 M K2SO4. 6. Calculate the solubilities of the following compounds in a 0.0333 M solution of Mg(ClO4)2 using (1) activities and (2) molar concentrations: (a) AgSCN. (b) PbI2. 7. Calculate the solubilities of the following compounds in a 0.0167 M solution of Ba(NO3)2 using (1) activities and (2) molar concentrations: (a) AgIO3. (b) Mg(OH)2. Part 3 Solving Equilibrium Problems for Complex Systems 1. Write the mass-balance expressions for a solution that is (a) 0.2 M in HF. (b) 0.10 M in H3PO4. (c) 0.20 M in Na2HPO4. (d) 0.0500 M in HClO2 and 0.100 M in NaClO2. 2. Calculate the molar solubility of BaSO4 in a solution in which [H3O+] is (a) 3.5 M. (b) 0.080 M. Ksp (BaSO4) = 1.1 × 10–10, H2SO4 (Ka1: large, Ka2: 1.2 x 10-2) 3. Dilute NaOH is introduced into a solution that is 0.050 M in Cu2+ and 0.040 M in Mn2+. Ksp of CuOH)2 = 4.8 × 10-20, Ksp of Mn (OH)2 2 × 10-13 (a) Which hydroxide precipitates first? (b) What OH- concentration is needed to initiate precipitation of the first hydroxide? (c) What is the concentration of the cation forming the less soluble hydroxide when the more soluble hydroxide begins to form? 4. Silver ion is being considered for separating I- from SCN- in a solution that is 0.040 M in KI and 0.080 M in NaSCN. Ksp of AgI: 8.3 x10-17 M, Ksp of AgSCN: 1.1 x10-12 (a) What Ag+ concentration is needed to lower the I- concentration to 1.0 x 10-6 M? (b) What is the Ag+ concentration of the solution when AgSCN begins to precipitate? (c) What is the ratio of SCN- to I2 when AgSCN begins to precipitate? (d) What is the ratio of SCN- to I2 when the Ag+ concentration is 1.0 x 10-3 M? 5. In contrast to many salts, calcium sulfate is only partially dissociated in aqueous solution: The solubility-product constant for CaSO4 is 2.6 x 10-5. Calculate the solubility of CaSO4 in (a) water and (b) 0.0100 M Na2SO4. In addition, calculate the percent of undissociated CaSO4 in each solution.