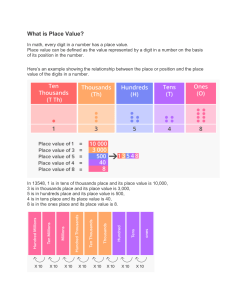
QoD 1. Al2(SO4)3(aq) + Ca(NO3)2(aq) -- > Al(NO3)3(aq) + CaSO4(p) Does this obey the law of conservation? Identify a reactant, product, coefficient and subscript What does the ‘(p)’ mean? How many oxygen atoms are on the product side? 2. How can you tell if a chemical change occurred? Breakout Peardeck Matter and its Changes Review States of matter 1. The state(s) of matter in which particles are touching and therefor cannot be compressed is/are ___________ 5. The state(s) of matter in which particles can be compressed is/are ___________ 2. The state(s) of matter in which particles are in a fixed position and vibrating is/are _________________ 6. The state(s) of matter in which particles take the shape of its/their container is/are ___________ 3. The state(s) of matter in which particles have a definite shape is/are _________________ 7. Convert 400 cm to m: 4. The state(s) of matter in which particles have enough energy to flow is/are _________________ The first digit of the code is the number of gasses, the second digits is the number of liquids, the third digit is the number of solids, and the fourth digit is the answer from #7. __ __ __ __ Properties of matter 1. Identify the following properties as (P)hysical or (C)hemical: 3. Identify the following CHANGES as (P)hysical or (C)hemical: __ Color __ Flammable __ Volume __ cutting paper __ dissolving sugar__ corroding __ Density __ Mass __ Digestible __ rusting 2. Identify the following properties as (I)ntesive or (E)tensive: __ Color __ Flammable __ Volume __ Density __ Mass __ Digestible __ melting __ boiling 4. What is the density of an object whose mass is 4g and volume is 2mL? The code is the number if P’s from #1, the number of E’s from #2, the number of C’s from #3, the answer from #4, followed by the color found in the first box. __ __ __ __ __ Matter – Mixtures vs Pure substances 1. Matter is anything with _______ and volume. 4. A mixture is two or more pure substances _________ combined. 2. Pure substances can either be an _________ or coumpound 3. Classify the following as an (E)lement or (C)ompound: __ H2 __ H2O __ O2 __ CO2 __ Au __ Na The first digit of the code is the first letter from #1, the second digit is the first letter from #2’s first answer, the third digit whichever has E or C from #3, the fourth digit is the firs letter of the answer from #4, and the fifth digit is color from the second box. __ __ __ __ __ Matter – Mixtures 1. Mixtures and be classified as homogeneous or heterogeneous based on the number of _____________ 4. State the number of phases seen here: 1 = star 2= triangle 2. A phase is a region of ____________ composition in a mixture. 3= arrow 4 = circle 5 = square 3. Classify the mixtures as Ho(m)ogeneous or He(t)erogeneous: __ Kool aid __ Chicken Soup __ Spaghetti __ Salt water __ Oil and water __ Salad The first digit of the code is the first letter from #1, the second digit is the first letter from #2, the third digit is the whichever has more from #3 (t or m), the fourth digit is the answer from #4, and the fifth digit is letter from the fourth box. Matter – Chemical Changes 1. A chemical change differs from a physical change because a chemical change produces matter with a different _____________ than what was started with. 4. NaOH + Cu(NO3)2 --> NaNO3 + Cu(OH)2 How many Oxygens are on the reactant side of the reaction? 3 = triangle 4 =r. arrow 5= square 6 = l. arrow 7= square 2. 3. What are the evidences that a chemical change occurred? NaOH + Cu(NO3)2 --> NaNO3 + Cu(OH)2 Does the reaction obey the law of conservation? If yes, the digit is triangle. If no the digit is circle The first digit of the code is the first letter from #1, the second digit is the number of evidences from #2, the third digit is answer from #3, the fourth digit is the answer from #4, and the fifth digit is shape from the fourth box. __ __ __ __ ___