History of the Atom: Timeline of Atomic Theory
advertisement

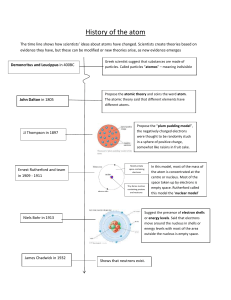
History of the atom The time line shows how scientists’ ideas about atoms have changed. Scientists create theories based on evidence they have, but these can be modified or new theories arise, as new evidence emerges Demoncritus and Leucippus in 400BC John Dalton in 1805 JJ Thompson in 1897 Ernest Rutherford and team in 1909 - 1911 Greek scientist suggest that substances are made of particles. Called particles ”atomos” – meaning indivisible Propose the atomic theory and coins the word atom. The atomic theory said that different elements have different atoms. Propose the “plum pudding model”, the negatively charged electrons were thought to be randomly stuck in a sphere of positive charge, somewhat like raisins in fruit cake. Mostly empty space containing electrons Tiny dense nucleus containing protons and neutrons Niels Bohr in 1913 James Chadwick in 1932 In this model, most of the mass of the atom is concentrated at the centre or nucleus. Most of the space taken up by electrons is empty space. Rutherford called this model the ‘nuclear model’ Suggest the presence of electron shells or energy levels. Said that electrons move around the nucleus in shells or energy levels with most of the area outside the nucleus is empty space. Shows that neutrons exist.