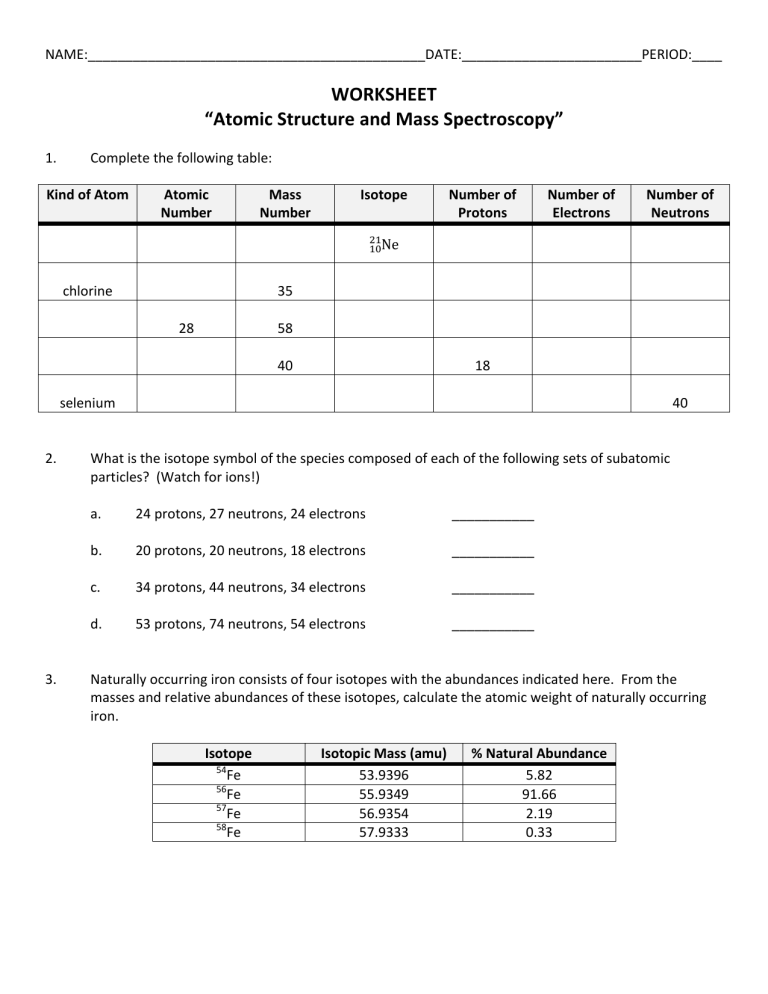
NAME:_____________________________________________DATE:________________________PERIOD:____ WORKSHEET “Atomic Structure and Mass Spectroscopy” 1. Complete the following table: Kind of Atom Atomic Number chlorine Mass Number Isotope Number of Protons Number of Electrons Number of Neutrons 35 28 58 40 18 selenium 2. 3. 40 What is the isotope symbol of the species composed of each of the following sets of subatomic particles? (Watch for ions!) a. 24 protons, 27 neutrons, 24 electrons ___________ b. 20 protons, 20 neutrons, 18 electrons ___________ c. 34 protons, 44 neutrons, 34 electrons ___________ d. 53 protons, 74 neutrons, 54 electrons ___________ Naturally occurring iron consists of four isotopes with the abundances indicated here. From the masses and relative abundances of these isotopes, calculate the atomic weight of naturally occurring iron. Isotope 54 Fe 56 Fe 57 Fe 58 Fe Isotopic Mass (amu) 53.9396 55.9349 56.9354 57.9333 % Natural Abundance 5.82 91.66 2.19 0.33 4. Determine the atomic mass and the identity of the elements which have the following atomic spectra. a. b. Atomic Mass = ___________ Identity of Element: _______________ Atomic Mass = ___________ Identity of Element: _______________ R1. Equal volumes of equimolar solutions of ammonia and hydrochloric acid are combined. ____________ Indicate whether the resulting solution is acidic, basic, or neutral. Explain. AP Free Response In an experiment, a student is given 2.94 g of a mixture containing anhydrous MgCl 2 and KNO3. To determine the percentage by mass of MgCl2 in the mixture, the student uses excess AgNO3(aq) to precipitate the chloride as AgCl(s). a. Starting with the 2.94 g sample of the mixture dissolved in water, briefly describe the steps necessary to quantitatively determine the mass of the AgCl precipitate. b. The student determine the mass of the AgCl precipitate to be 5.48 g. On the basis of this information, calculate each of the following. i. The number of moles of MgCl2 in the original mixture. ii. The percent by mass of MgCl2 in the original mixture.




