Uploaded by
Laura Camp
Periodic Table Worksheet: Elements & Properties

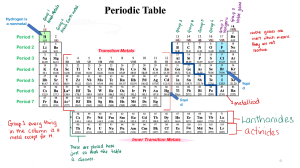
Getting to Know the Elements Color and label your periodic table according to the following instructions. 1. 2. 3. 4. Label the horizontal rows 1-7. Number the vertical columns 1-18 if not already done. Outline (do not shade) all the metals with red. Alkali metals a. Color them pink. DO NOT COLOR HYDROGEN. Leave it white. b. Put a charge of 1+ above the group. 5. Alkaline earth metals a. Color them light brown. b. Put a 2+ charge above the group. 6. Transition metals – shade them yellow 7. Put diagonal lines on iron, cobalt, and nickel to show that they are magnetic. 8. Color the rest of the remaining metals in groups 13-16 orange. 9. Color the metalloids (semiconductors) red. 10. Outline (do not shade) the nonmetals in green. 11. Noble gases – color them purple 12. Halogens – color them light blue 13. Label radioactive elements with a ∆. All elements with an atomic number 83 or greater. 14. Label all synthetic (man-made) elements with this symbol ⃝ . All elements with an atomic number 93 or greater. 15. Tc and Pm are synthetic and radioactive. Label them. Questions and fill-in-the-blank. 1. The periodic table of elements arranges elements by increasing _________________ number and shows repeating patterns of electron configuration. 2. Vertical columns are called _______________ or families. Because they have similar valance electron configurations (arrangements), they have similar chemical properties. 3. List 3 characteristics of metals. a. b. c. 4. Alkali metals are in group ____ and have ____ valence electron(s) and form ions with a charge of ____. 5. Three characteristics of alkali metals a. They are ____________ and shiny. b. They are _____________________ reactive; reacts violently with water. c. They are not found in _______________ uncombined. 6. Alkaline earth metals are in group ________. a. Have a _____ valence electrons and a charge of ______. b. Are _________ reactive than alkali metals, but still not found in ______________ uncombined. c. List 3 examples and where you might find them. 1) 2) 3) 7. Transition metals are in groups _______ through ________ and form ________________ ions. 8. List 3 common examples of transition metals and their uses. a. b. c. 9. Metalloids (semiconductors) are able to conduct heat and electricity under certain _____________________________. 10. Nonmetals are different from metals in that they do not conduct ____________ or _________________________. 11. Noble gases are in group _______ and have ________ outer energy levels and are not reactive. 12. Halogens are in group ______ and have ______ valence electrons. They tend to form ions with a _____ charge. They are _________ reactive. 13. This symbol means ____________________________. 14. Synthetic elements are ________-made and do not exist naturally in the environment. 15. What connection do you see between synthetic elements and radioactive elements? 16. Hydrogen is a non-metal but is placed in group one because it has _____ valence electron(s) and forms ions with a ______ charge. 17. Make a key for your table explaining the colors and symbols.