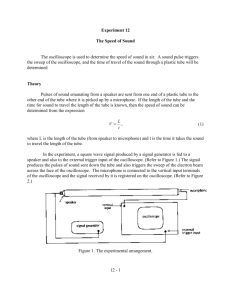
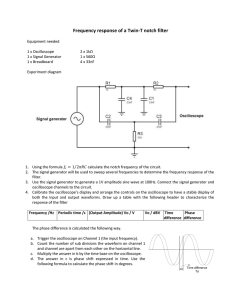
Experiment 8 Measurement of the heat-capacity ratio of gases by the speed of sound method Introduction In this experiment you will measure the speed of sound in three gases, and use Eq.(1) to deduce their heat-capacity ratios. The speed of sound, u, in a gas can be shown to be γRT u M 1 2 (1) where Cp/Cv is the heat-capacity ratio, M is the molecular weight, R is the gas constant and T is the absolute temperature. Because sound propagates in gases through collisions, it is not surprising that Eq.(1) closely resembles the expression for the mean speed of molecules derived from the gas kinetic theory: <v>=(8RT/M)1/2. Please refer to ref. (1) or (2) for a detailed derivation of Eq.(1). Before you come to the laboratory, you may also want to review your P. Chem. textbook about (i) the relation between Cp and Cv, (ii) the equipartition of energy theorem and how it is used to predict Cv.A sketch of the apparatus that you will use is shown in Fig. 1. Pressure gauge To gas manifold Microphone Speaker AMP 1.203 CH1 CH2 Function generator Figure 1. Experimental setup for measuring the speed of sound. The apparatus consists of a stainless steel tube, a function generator and an oscilloscope. A 59 small loud speaker is mounted on one end of the tube and a small microphone is mounted on the other end. The distance between the speaker and microphone is 54.5 cm. The function generator generates an alternating sine-wave electric signal that is used to drive the loud speaker, producing sound waves at precisely the same frequency. A built-in frequency meter in the function generator measures the frequency and shows the reading on the front-panel LED display. This signal is also used to drive the horizontal (x) sweep of the oscilloscope. Thus, the horizontal motion of the electron beam in the oscilloscope is in phase with the sound wave emanating from the speaker in the tube. The sound generated by the speaker travels down the tube and strikes the microphone. The sound waves are converted into an alternating electric signal by the microphone, and this signal is then amplified and used to drive the vertical sweep of the oscilloscope. Thus the vertical motion of the electron beam in the oscilloscope is in phase with the sound wave as they pass the microphone. However, the phase of the sound waves impinging upon the microphone may or may not be the same as those at the speaker. In general there will a phase delay, , between the sound waves at the speaker and microphone. Likewise, the same phase delay is also present between the electric signal driving the x and y sweeps of the oscilloscope. The relative phasing of the two electrical signals determines the shape of the pattern seen on the oscilloscope. Suppose that the frequency of the sound wave generated at the speaker is f, then the signal driving the horizontal sweep of the oscilloscope can be expressed as x=A sin (2f t) (2) Likewise, the sound wave impinging on the microphone is transformed into an electrical signal that is used to drive the vertical sweep and can be expressed as y=B sin (2f t+) (3) , where = 2d/ is the phase delay of the sound wave impinging upon the microphone with respective to that emitting at the speaker. The patterns you will observe on the oscilloscope are special cases of the Lissajous figures. Here are some representative patterns for equal amplitude signals, i.e. A=B, at =0, /4, /2, 60 = 0 = /4 = /2 = (a) (b) (c) (d) Figure. 2 Lissajous figures for various values of the phase delay between signals of equal amplitude. Try to prove to yourself mathematically that each special case gives the corresponding Lissajous figure. Note that the amplitude of the two signals, A and B, are not equal most of the time in the experiment. Try to deduce the shapes of the Lissajous figures for the above cases when A≠B. This will help you understand the patterns you will observe on the oscilloscope during the experiment. When =0, we say the x and y waves are exactly in phase; when =, we say the two waves are 180 out of phase. In these two cases the wavelength of the sound wave must satisfy n d , n=1,2,3 2 (4) , where is the sound-wave wavelength, d is the distance between the speaker and microphone, and n is a positive integer number. The sound-wave frequency and wavelength are related by f =u (5) Combining Eq(4) and (5), one obtains f u n 2d (6) In this experiment, you will tune the frequency (and thus the wavelength) while observing the patterns on the scope. When the condition of Eq(4) is met at a certain frequency, you observe a tilted straight line similar to that depicted in Fig. 2(A) or (D). A series of successive frequencies satisfying such conditions are taken and used to derive the speed of sound. Experimental procedures You will measure the speed of sound in three gases: N2, CO2 and He. First, turn on all electronic instruments, i.e. the function generator, the oscilloscope and the audio amplifier. Make sure they are connected as shown in Fig. 1 and all connections are good. (1) Make sure the function generator outputs sine waves. (the function generator can also output 61 square and saw-tooth waveforms) If not, press the “sine” button on the front panel. (2) Make sure the oscilloscope is in the X-Y mode. If not, press the “X-Y” button. This should switch the scope from the internal time-sweeping mode, i.e. Y-t, to the X-Y mode. You should see a “X-Y” sign on the upper-left corner of the screen when the oscilloscope is in the X-Y mode. (3) For N2 and CO2, set the function generator coarse frequency at 1kHz by pressing the “1k” button on the front panel. For He, set the coarse frequency at 10 kHz. (4) Now, evacuate the tube. Your TA will instruct you how to operate the vacuum manifold. Keep pumping for at least 2 min. or until the pressure gauge goes all the way down. (5) Slowly introduce the gas to be studied into the tube. The pressure should be slightly above 1 atm. It might be easier to begin with N2 or CO2. (6) Now, slowly turn the frequency-adjusting knob while observing the patterns appearing on the scope. You should see some Lissajous figures that change their shapes rapidly as the frequency is tuned. Make sure the frequency is in the range between 1.1 to 12 kHz. Sound waves with frequencies outside this range, either too low or too high, cannot be faithfully reproduced or amplified by the instruments used here. You should also keep in mind that it is difficult to produce mathematically perfect Lissajous patterns in this experiment, because the speaker, microphone and amplifier all distort the signal to some extents. The amplitude of signal coming from the microphone also varies with the frequency; this is because the speaker and the microphone only works best in a certain frequency range and the amplifier has a finite bandwidth. When necessary, adjust the gain-control knob for CH1 or CH2 to make the patterns look similar to those depicted in Fig. 2, i.e. A=B. (7) For N2 and CO2, slowly tune the frequency from 1.1 kHz to 3.33 kHz and determine the successive “in phase” and “180 out of phase” frequencies, i.e. when the pattern on the scope becomes a tilted straight line. For He, the frequency range should be from 3.3 kHz to 8kHz, and you might have to increase the vertical gain of the oscilloscope for this gas. (8) Repeat steps (5) – (8) for the other two gases. 62 Data Analysis (1) N2 f(kHz) n CO 2 f(kHz) n He f(kHz) n (2)For each gas studied, plot the recorded frequencies against n’s, which can be an arbitrary set of successive integer numbers, beginning with the low-frequency data. This plot should give a straight line with a slope of u/2d, as indicated in Eq(6). Please pay attentions to the units that you use. Use a commercial program, such as MS excel, Origin, etc., to perform a linear least square fit to obtain the slope, and then derive the speed of sound. (3)Use Eq. (1) to calculate the heat-capacity ratio for each gas. Discussion and Questions 1. Compare your speed of sound values with those in the literatures and report the accuracy of your measurement. 2. Compare your experimental heat-capacity ratios with those predicted by the equipartition of energy theorem, and make any deductions you can about the presence or absence of rotational and vibrational contributions. 3. How would the theoretical heat-capacity ratio of CO2 be affected if the molecule were nonlinear? References: 1. A. M. Halpern, “Experimental Physical Chemistry: a laboratory textbook,” 2nd ed., Experiment 2, Prentice Hall (1997). 2. C. W. Garland, J. W. Nibler and D. P. Shoemaker, “Experiments in Physical Chemistry,” 6th ed., Experiment 3, McGraw-Hill (1996). 3. M. A. Duncan, “Physical Chemistry Instrumentation and Experiments,” p.45, University of Georgia (1985). 本教學實驗由教育部”提升大學基礎教育計劃”補助,硬體系統由李以仁先生(清大化學 98 級)協助建立,鄭博元教授指導。 63