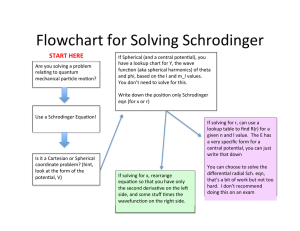
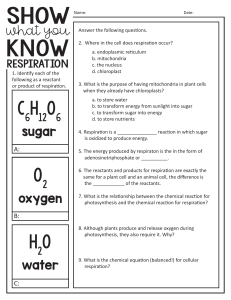
Guided Reading Chapter 17 Sec ons 1 and 2 1.Physical and chemical changes involve ______________. 2.A chemical reac on is the process of breaking molecular bonds and reforming them into a new ________________. a.atom b) substance c) element 1.List a few ways that would lead you to believe a chemical reac on had occurred. 1.What are reactants and products in a chemical reac on? 1.Complete the following table: Symbol Meaning (s) Substance is a liquid (g) Substance is dissolved in a solu on 1.The statement, “the total mass of the products must equal the total mass of the reactants” is known as the __________________________________________. ti ti ti ti ti 2.The _____________ mass is the sum of the atomic mass units of all the atoms in a chemical formula. a.formula b) isotope 1.A mole of any substance is equal to a.6.02 X 1022 b) 6.02 X 10-23 c) 6.02 X 1023 1. c) molar 2.Why is it useful to write chemical equa ons? 1.When balancing a chemical equa on you may only change the ________________, not the subscript. 2.Can you balance this equa on using the rules on page 394–395? _____Ca + _____O2 _____CaO 1.Can you balance this equa on using the rules on page 394-395? ti ti ti ti _____Na2O + _____H2O _______NaOH