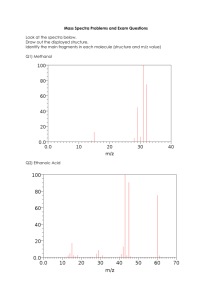
Mass spectrometry (MS) Review homework (from CHEM2411L) PP. 7-13 Due Wednesday, August 18. Bring to class Purpose: In this lab you will learn how to analyze mass spectra of organic compounds. From mass spectra, you can learn the molecular mass, elucidate possible chemical formula, and some information about functional groups. Mass spectrometry is a powerful analytical tool applicable across diverse fields, including forensic, environmental, pharmaceutical, and clinical analysis. Having a working experience with mass spectrometry will broaden your employability in local industrial and analytical laboratories. Spectroscopy is especially powerful when multiple methods are combined. You will practice combining mass and IR spectra to identify a compound. This assignment will help you practice the following skills: • Convert molecular structure into formula, and calculate molecular ion mass • Read the mass spectra to find the molecular ion peak (M+) • Identify fragment peaks, and dissect molecules to find how some fragments form • Finding a M+1 peak and estimate the number of C atoms in the formula • Finding a M+2 peak to see whether Cl or Br is in the formula • (Review) Infrared (IR) spectroscopy: determining the presence or absence of certain covalent bonds or functional groups • Integrating the information derived from IR and mass spectra to identify a compound This assignment will help you gain (or review) the following knowledge: • The meaning of m/z (mass over charge ratio) • How ionization occurs in the mass spectrometer, and how the mass over charge is determined • Difference between (average) atomic mass and exact atomic mass • How molecular ion peak and fragmentation peaks form, and how to identify them • Isotopes and how they affect the sizes of M+1 and M+2 peaks (focus on C, Cl and Br) o 13C/12C ~1.1%, and M+1 peak is # C x 1.1% of the M+ peak o 37Cl/35Cl ~33%, and M+2 peak is about 1/3 of the M+ peak o 79Br/81Br ~ 98%, and M+2 peak is about the same size as the M+ peak -1- Theory and Background Mass spectra and fragmentations Mass spectrometry (MS) is an analytical technique to detect the molecular mass or mass of molecular fragments. Most MS instruments have the same three essential components: (i) ionization of neutral molecules, (ii) separation of ions by acceleration under electric field toward the detector, and (iii) detection and quantification of the separated ions. The most common ionization method used in organic chemistry is electron ionization (EI). In the ionization source, the high-energy electron beam knocks off a valence electron from the molecule to form a radical cation. Knocking off an electron to make a +1 ion can be a harsh process. This harsh treatment often results in a broken bond, generating two smaller pieces, a +1 ion and a neutral fragment. (Note that only the ion is accelerated and detected.) For example, the EI-MS spectrum of acetone is shown in Figure 1. The horizontal axis is mass over charge ratio, m/z. In this activity we will consider only the mass spectral data of +1 ions. This means we can assume that m/z = mass. m/z Figure 1. Mass spectrum of acetone Acetone, whose formula is C3H6O, has a molecular ion mass of 12x3 + 1x6 + 16x1 = 58. Note that this mass is calculated using only the atomic mass of the most abundant isotope of C (12), H (1), and O (16). You see a peak at m/z = 58, which is the molecular ion peak, which formed upon a loss of one electron upon ionization process (Figure 2). Figure 2. Molecular ion formation Figure 3. Fragmentation -2- You also see the fragment peaks of acetone at m/z = 15 and m/z = 43 in Figure 1, as a result of the fragmentation of the acetone molecular ion (Figure 3). The Electron Impact ionization method is energy intensive, and often cause the breaking of covalent bonds. As a result, the molecular ion (which is a radical cation) breaks down to fragments, one carrying the + charge, and one carrying the radical. Only the cation fragments are seen in the mass spectrometry. But the + charge and radical can be on either of the fragments. The fragmentation analysis (see insert in Figure 1) can be done by simply “chopping” or “dissecting” one of the single bonds (do not “chop” multiple bonds because they are harder to cleave) on the molecular structure, and calculate the fragment mass of both sides. Then you can see if there are any matching fragment ions in the mass spectrum. In Figure 1, the m/z = 15 and m/z = 43 are explained by the dissection of the carbonyl C-CH3 bond (Figure 3). See more examples of the fragmentation analysis below. Aliphatic hydrocarbons often show a series of fragments at different C-C bonds. Figure 4 shows the fragmentation analysis of 2-methylpentane on its mass spectrum. Assume that most observed ions are the result of one bond cleaving, and not a result of sequential bonds cleavage. C3H7+ C5H11+ CH3+ C2H5 + C4H9+ M+ Figure 4. Mass spectrum (EI) of 2-methylpentane and fragmentation analysis The mass spectrum of 2-nitro-1-napthol and the fragmentation analysis is shown in Figure 5. Loss of substituents -OH (-17) and –NO2 (-46) occurs readily and the residual fragments are seen at m/z = 172 and 143. Aromatic rings are relatively difficult to break down. M+ m/z = 143 -46 -17 Figure 5. Mass spectrum (EI) of 2-nitro-1-nathtol -3- MS Isotopic patterns You will learn three atoms that have distinguishable isotopic patterns: C, Cl, and Br. They have a relatively high abundance of minor isotopes: see Table 1. Mass spectrometry can distinguish molecules containing different isotopes, because they have different masses. For example, a molecule of 12CH4 shows m/z =16 peak, whereas 13CH4 shows m/z = 17 peak. Table 1. Atoms that contribute to M+1 or M+2 isotopic peaks Atom C Cl Br Atomic mass (average) 12.011 35.455 79.9 Isotope Exact atomic mass (amu) Abundance Relative ratio 12 13 C 12 C 98.9% 13 (+1) 1.1 % 100 1.1 35 37 Cl Cl 35 79 81 Br 79 Br 99.6% 37 (+2) 0.4% 99.8% 81 (+2) 0.2% 100 32.5 100 98 You will NOT worry about the minor isotope’s contribution by H, N, O, because they are >99.6% single isotope, and their second most abundant isotopes are too minor to observe (Table 2). Whenever you calculate a molecular mass containing H, N, or O, use their most abundant mass in simple integer (H = 1, N=14, O=16). Table 2. Atoms with one single dominant isotope (M+1 peaks not observable) Atom H N O Atomic mass (average) 1.0078 14.0067 15.9994 Isotope Exact atomic mass (amu) Abundance 1 2 14 15 2 14 15 16 18 0.02% 99.6% 0.4% 99.8% 0.2% H H (aka deuterium, D or 2D) 1 99.98% N -4- N 16 O 18 O Carbon-containing molecules: For every 100 12C atoms, there are 1.1 of 13C isotopes. Therefore, carbon-containing ions always have a small “M+1” peak. For a formula containing multiple C atoms, the chance that one of the C atoms being 13C increases by the number of C atoms. For example, if a formula is CnHxNy, then the there is n x 1.1% chance that one of the C atoms is 13C. Therefore, any Ccontaining mass peak will have n x 1.1 % height of M+1 peak (n = number of C in the formula) next to the main peak (that consists of only 12C). For example, find the molecular ion peak of anthracene in its mass spectrum in Figure 6 (anthracene, C14H10, exact molecular mass 178). Do you see the M+ peak (m/z = 178) and M+1 peak (m/z = 179)? Anthracene molecule has 14 C atoms, M+1 peak is predicted to be 14 x 1.1% = 15.4 %. Does the M+1 peak size roughly match the prediction? Mark M+ and M+1 peaks on the spectrum! m/z Figure 6. Mass spectrum (EI) of anthracene -5- Cl-containing molecules: Chlorine’s most common isotope is 35Cl, but the next most common isotope is 37Cl (32.5% relative to 35Cl). You would see roughly 3:1 ratio of M and M+2 peaks (→) in a molecule containing one Cl atom. For example, please see the mass spectrum of 2-chloropropane (Figure 7). The main M peak at m/z = 78 contain the most abundant 35Cl, but it is accompanied by m/z = 80 peak at about 1/3 intensity. The latter is from 37Cl-containing M+2 peak. One Cl M + 2-Chloropropane M M+2 Figure 7. Mass spectrum of 2-chloropropane, showing the M+2 isotopic pattern Br-containing molecules: One Br Bromine has roughly 1:1 ratio of 79Br and 81Br (100 : 98). If you see a twin-tower-like peaks two units apart, it is likely the M : M+2 peaks at approximately 1 : 1 ratio due to the molecular ion M contains one Br atom. For example, see the mass spectrum of 1bromopropane (Figure 8): the m/z = 122 and 124 peaks at roughly 1:1 ratio are the M and M+2 peaks. M 1-Bromopropane M M+2 (-Br) Figure 7. Mass spectrum of 2-chloropropane, showing the M+2 isotopic pattern -6- + M+2 ~1/3 M+2 Name___________________________________ Mass spectrometry Review Homework Complete by the beginning of next class: August 18, 9:30am Bring to class 1. See the mass spectrum of methylcyclonexane and answer the questions. (i) What is the molecular formula of the molecule? (e.g. C3H5N) __________________ (ii) Calculate the formula mass using the most abundant atomic masses (e.g. C = 12, H = 1, N = 14, O = 16). Show calculations below. (iii) On the spectrum above, label the molecular ion peak [M]+ with an arrow, and write the formula and m/z value beside it (e.g. [M]+, C4H10+, m/z = 54). (iv) Label the M+1 isotopic peak on the spectrum. (v) Which isotope is responsible for the M+1 peak? _______ (vi) Circle the most intense fragment peak, and show below the “dissection” of the molecule that is responsible to give the fragment. Show the formula and mass of the fragment as well. (Tip: draw all “C” and “H” atoms on the structure to count atoms correctly.) -7- 2. For the mass spectrum of Z-2-hexene shown below, write the formula and calculate the formula mas, and answer the following questions. (i) Draw the molecular structure of Z-2-hexene. (ii) What is the molecular formula? __________________ (iii) Calculate the exact formula mass based on the atomic masses of the most abundant isotopes. Show calculations. (iv) On the spectrum, label the molecular ion peak ([M]+) and the M+1 peak. () (v) Redraw the molecule below, and show the fragmentation analysis to account for the fragment peaks at m/z = 15, 27, 29, 41, 55, 69 (indicated with arrows in the spectrum). -8- Commented [WU1]: Delete this 3. For the mass spectra in the next page, select a matching compound from the choices given below. In addition, (i) Mark M+ peaks and M+1 (if no halides) or M+2 peaks (if containing Cl or Br), and (ii) Identify the fragment peak(s) marked with an arrow. Choices: Show work ↓ (Structure, formula & formula mass, fragmentation analysis) -9- (3 continued) Show work ↓ (Structure, formula & formula mass, fragmentation analysis) - 10 - 4. The following three mass spectra belong to the isomers shown below. Based on the fragmentation pattern, assign the molecules to the spectra. Justify your answer by clearly showing the fragmentation analysis, matching the fragment mass to mass peaks present. Show work ↓ - 11 - 5. MS and IR combined problems Based on the mass and infrared (IR) spectra, choose the correct structure for each unknown from choices below. Show all work and justify your answer. ➢ From the IR spectrum, you can find functional groups to narrow down the candidates. ➢ From the mass spectrum, you can find the molecular mass, and the presence or absence of Cl or Br. ➢ Fragment peaks may or may not be useful, as there are possibly many fragments from the mechanisms that you have not learned about. (5-1) Unknown A Transmittance (%) IR spectrum of A Wavenumber (cm-1) Show all work. Mass spectrum of A - 12 - Transmittance (%) (5-2) Unknown B IR spectrum of B Wavenumber (cm-1) Show all work. Mass spectrum of B (End of In-Class Worksheet) - 13 - - 14 -