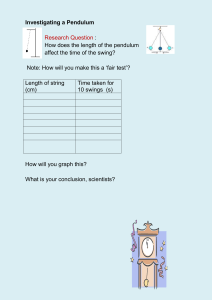
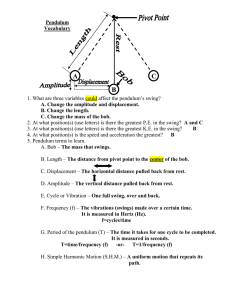
Yew Chung International School of Shanghai – Gubei Campus Science Department IGCSE Coordinated Sciences (0654) & Combined Science (0653) Guide for students Name________________ Albert Einstein "Not everything that counts can be counted, and not everything that can be counted counts." (Sign hanging in Einstein's office at Princeton) -2- Contents Useful Websites 5 IGCSE Course Overview 6 Course Assessment 7 Plagiarism 8 Referencing 8 YCIS Science Safety Rules 11 Assessment & Grade Thresholds 12 Guide to Writing Experimental Reports 13 IGCSE Example Lab 1: Pendulum Investigation 18 Symbols, Units and Definitions of Physical Quantities 35 Periodic Table 36 Notes for use in Qualitative Analysis 37 Glossary of Terms Used in Science Papers 38 Studying Effectively 39 Glossary 40 -3- Ernest Rutherford Niels Bohr “If your result needs a statistician then you should design a better experiment” “An expert is a man who has made all the mistakes which can be made in a very narrow field” Sir Isaac Newton Dorothy Hodgkin “If I have ever made any valuable discoveries, it has been due more to patient attention, than to any other talent” “I was captured for life by chemistry and by crystals” Dmitri Mendeleev Gregor Mendel "The elements, if arranged according to their atomic weights, exhibit an apparent periodicity of properties." “The value and utility of any experiment are determined by the fitness of the material to the purpose for which it is used, and thus in the case before us it cannot be immaterial what plants are subjected to experiment and in what manner such experiment is conducted.” -4- Useful Websites 1. http://www.cambridgestudents.org.uk The official CIE website which contains: Past papers, revision tips, study checklists, exam advice, online competitions. 2. http://www.rsc.org/education/teachers/learnnet/alchemy/index2.htm From the RSC - good for resources including videos on industrial process 3. http://www.rsc.org/chemsoc/timeline//pages/timeline.html 4. http://www.s-cool.co.uk/default.asp 5. http://www.bbc.co.uk/schools/gcsebitesize/science/ BBC Bitesize Revsion → general revision → tests 6. http://www.channel4.com/science/ Very good site; also has a question facility called "Ask an Expert"! -5- IGCSE Course Overview Both Coordinated Sciences and Combined Science cover all three sciences: Biology, Chemistry and Physics. One third of each course is spent on each science. Both courses use the same textbooks and every year 10 student and every year 11 student will be issued with both books at the start of the year. The text books are: Balanced Science 1 (ISBN 0521599792) and Balanced Science 2 (ISBN 0521599806) published by Cambridge University Press. Other texts are used to support these text books. The units of work in years 10 & 11 cover all areas needed to progress to an advanced course of study (IB Diploma) in one of the sciences. All students are taught the extended/supplement syllabus for the duration of the course. Year 11 Year 10 Biology Chemistry Physics B1 – The principles of biological classification and the diversity of organisms B2 – Cellular organization and function B3 – Support and movement B4 – Photosynthesis B5 – Gaseous exchange in animals B6 – Transport systems B7 – Respiration B8 – Diet and health B9 – Digestion B10 – Responding to changes in the environment B11 – Homeostasis C1 – The elements of chemistry C2 – Classifying the elements C3 – Petrochemicals C4 – Chemicals from plants C5 – Materials and structures C6 – Oxidation and reduction C7 – Ions and electrolysis C8 – Solvents and solutions C16 – Metals and alloys C17 – Atoms, bonding and the periodic table P1 – The strength of solids P2 – Particles in motion P3 – Motion P4 – Force and motion P5 – Energy transfer P6 – Transferring energy by heating P7 – Using electricity P8 – Energy and electricity P9 – Waves P10 – Light and sound P11 – Making use of waves B12 – Reproduction B13 – Human reproduction B14 – Inheritance B15 – Evolution B16 – Organisms in their environment B17 – Cycles and the effects of humans on their environment C9 – Acids and alkalis C10 – Soil, rocks and reaction rates C11 – Fertilizers C12 – Dyes and drugs C13 – Colloids C14– Fuels C15 – Batteries P12 – Kinetic energy and momentum P13 – Gravity P14 – Magnetism and electricity P15 – Communication P16 – Electrons P17 – Radioactivity P18 –Energy resources P19 –Energy distribution P20 –Electronics -6- Course Assessment Both courses last for two years. Students are continuously assessed through coursework, homework, experimental work and end of unit tests. The final examinations at the end of year 11 are externally assessed. All students will sit three exams: Paper 1 and Paper 2 OR 3, and Paper 6. All students sit these two papers: Paper 1 (multiple choice) • 40 multiple choice questions based on core material. • 45 minutes. • 40 marks total. • Worth 30% of the final grade. • Period table is included. • Calculators are allowed. • Dictionaries of any sort (definition/treanslation or paper/electronic) are NOT allowed. and Paper 6 (alternative to practical) • Short answer questions based on experimental work. • 1 hour • 60 marks total. • Worth 20% of the final grade. • Paper translation dictionaries only are allowed. • Calculators are allowed. • Periodic table is NOT attached to the exam paper (not allowed). All students sit one of these papers (i.e. paper 2 OR paper 3). Which paper students sit is based on grades achieved and is determined near the end of the course. Paper 2 (short answer) • Short answer questions on core material. • 1 hour 15 minutes (Combined) OR 2 hours (Coordinated). • 100 marks total. • Worth 50% of the final grade. • Paper translation dictionaries only are allowed. • Calculators are allowed. or Paper 3 (short answer) • Short answer questions on extended material • 1 hour 15 minutes (Combined) OR 2 hours (Coordinated). • 100 marks total. • Worth 50% of the final grade. • Paper translation dictionaries only are allowed. • Calculators are allowed. • Periodic table is included. -7- 9th April 2008 Dear Parents, I write to you on a serious matter. The issue of plagiarism is becoming more common across Schools worldwide and I think it would be helpful if I set out what plagiarism is and why it is so important to avoid. A common definition of plagiarism is: “Plagiarism is the practice of claiming or implying original authorship of (or incorporating material from) someone else's written or creative work, in whole or in part, into one's own without adequate acknowledgement. Unlike cases of forgery (in which the authenticity of the writing, document, or some other kind of object itself is in question), plagiarism is concerned with the issue of false attribution.” (Wikipedia 2008) Plagiarism is essentially cheating. If a pupil uses someone else’s work without acknowledgement, he or she is implying that it is their own work – and that is not the case. This is not a minor matter. With the increased use of the internet and the increasing assumption by some students that their work must be ‘perfect’, a number of students simply search for content on the Internet and then ‘cut and paste’ paragraphs or whole articles which they then hand in as either their own work or as ‘research’. This practice is not acceptable. There is nothing wrong with using the comments of others or the ideas of others in your own work – but these must be properly acknowledged. There are a number of ways in doing this, and I cite a common one below: “God’s Word does more than give being. It sets limits and provides life. Apple seeds do not produce plum trees. They grow up into apple trees and they do this at the Word of the Lord.”1 If you look at the bottom of this page, you can see that the above quote is identified by the title of the book the author the publisher and date of publication the page number If you look at the beginning of my letter there is an alternative option of placing the source in brackets after the quote. Whatever choice a pupil makes, they should be consistent. A useful test is to check whether the information provided is enough for a reader to check the source without difficulty. Pupils often make two false assumptions when they think they need to plagiarize: The work submitted must be perfect. This is not the case. Homework or research should reflect the pupil’s best effort, but does not need to be perfect to gain high marks. A pupil has left it too late to write something themselves and decides that handing in a piece copied from the Internet is better than handing in no homework at all. This is not the case. Plagiarism creates a problem that is much larger than an apology to the teacher for late homework. If a pupil does submit plagiarized work, it is very likely to be detected. This is for the following reasons: the work might use language which is more advanced than the pupil normally uses in their work the ideas or concepts are too complex for the pupil or explained in a way that is different to the way the pupil normally explains things the question is not properly addressed and there is a lot of irrelevant material included a number of students might submit the same piece of work Schools also have access to an additional software system to which work is routinely submitted. Pupils should also be aware that teachers (and Co-Principals) have a great deal of experience in spotting plagiarism. 1 Reclaiming the Future of Christian Education, A.E.Greene, ACSI 1998, page 83 -8- The penalties for plagiarism are severe. At a minimum, the work in question is not marked and given a zero grade. Other sanctions may also apply. If plagiarism is attempted in work submitted for an examination subject (such as at IGCSE or the IB), penalties include the loss of an entire examination subject (and in the case of the IB, the loss of the Diploma). In examination years, penalties can also be financial as the School needs to move a pupil from one examination unit to another and this cost will be passed on to parents. Plagiarism in later life (at university or in a profession) is not a risk worth taking either. Students lose their degrees, writers have had to withdraw books from circulation and even governments have had to apologize. Politicians have had to abandon their political careers when they were found to be using words taken from the speeches of others without acknowledgement. There is no need for plagiarism. We have been given the ability to think, decide and write for ourselves. Let us use these abilities. Yours, Dr Barkei Co-Principal -9- Marie Curie “Nothing in life is to be feared. It is only to be understood.” - 10 - YCIS Science Safety Rules Gubei Campus As part of the IGCSE Science Courses offered at YCIS you will be working in the laboratories regularly. Many laboratory activities require the use of hazardous chemicals and materials. To ensure that work in laboratories is done in a safe environment, the following rules must be followed: 1. You must follow all safety instructions stated by your teacher. This involves wearing safety glasses/goggles and lab coats. Sometimes additional safety requirements will be required. Long hair must be tied back and no open footware is allowed. 2. You must only do the experiment/procedure that you have been instructed to do. Do not touch any other objects/equipment/chemicals. 3. Books, purses, backpacks, etc. must be stored in an area designated by your treacher. 4. Do not do the following in a laboratory EVER: eat food, drink beverages, chew gum or run. 5. Work areas and equipment should be kept clean and tidy at all times. Bring only materials specified by your instructor to the work area. 6. Dispose of all waste materials in an appropriate manner as designated by your teacher. This means do not pour anything down the sink unless you are told to by your teacher. Do not return chemicals to their original containers unless you are specifically instructed to do so. 7. Read chemical labels very carefully. Make sure that you have the correct substance in the correct concentration. Check the label twice before removing any of the contents. Follow the instructor’s safety instructions for handling hazardous materials. 8. Never take chemicals, supplies, specimens, or equipment out of the laboratory without the knowledge and consent of your teacher. 9. Never enter a laboratory without the supervision of a teacher. 10. Never point the open end of a test tube being heated at yourself or others. 11. Always protect the balances/scales when weighing chemicals. 12. If you spill material clean it up immediately. 13. Used glassware goes in the dirty glassware container located in each laboratory. 14. Know the locations of fire extinguisher, fire blanket, eyewash, safety shower, and first aid kit. Accidents and Injuries 1. Report any accident (spill, breakage, etc.) or injury (cut, burn, etc.) to your teacher immediately. 2. Water spills on the floor need to be cleaned up immediately. 3. If a chemical should splash in your eye(s) or on your skin, immediately flush with running water from the eye wash/safety shower for at least 15 minutes. Notify your teacher immediately. You must also see the nurse (on the ground floor of A block). 4. Treat burns immediately by putting the burned area under cold water. You must also see the nurse (on the ground floor of A block). - 11 - IGCSE Experimental Programme All students are required to complete IGCSE experiment programme. This is structured to cover the scientific theory and techniques required by the syllabus. It is also designed to prepare students for the programmes of study at IB level. The laboratory work completed in years 10 and 11 counts for 20% of the final grade. Students complete two types of experimental reports: short answer and formally assessed experiments. Formally assessed reports are marked on four possible skill areas: 1. 2. 3. 4. Design (D) – Designing/planning the experiment Data Collection and Processing (DCP) – Collecting data in tables and presenting it as graphs Conclusion and Evaluation (CE) – Explaining and evaluating the results of your experiment Manipulative Skills (MS) – Working safety and as part of a team in the laboratory You could be assessed on all four criteria or one or two or three of the criteria. Your teacher will advise you prior to each lab which aspect you will be assessed on. Grade Thresholds – Coordinated Sciences Grade A* A B C D E F % 91-100 80-90 68-79 50-67 38-49 26-37 0-25 Report Comment Excellent Very good Good Satisfactory Mediocre Poor Very poor Grade A* A B C D E F % 85-100 70-84 55-69 40-54 30-39 20-29 0-19 Report Comment Excellent Very good Good Satisfactory Mediocre Poor Very poor Grade Thresholds – Combined - 12 - Guide to Writing Experimental Reports Design (D) 1 – Defining the problem and selecting the variables Total: /16 Formulate a focused research question (RQ). The research question must be short and concise (one sentence). It should contain the dependent and the independent variables. It should be written after the words: “Research Question” /2 Gives a qualitative hypothesis and justifies it with scientific reasoning and a calculation if appropriate. The hypothesis should be written after the words: “Hypothesis”. Below this comes the scientific reasoning and calculation if appropriate. At the minimum, your report should contain one internet reference and one book reference. /2 Identifies the key factors (variables) that should be varied or controlled. There are three classes of variables: independent (the one you change), dependent (the one you measure) and the control variables (the ones kept constant by you or are assumed to be constant such as external pressure on a calm day or the force of gravity). In this section you need to overview the possible investigations and then decide on the variables you will change (independent) and measure (dependent). See selecting variables below. 2 – Selecting variables 3– Developing a method for collection of data States the independent variable (the one you change). States the dependant variable (the one you measure). States the controlled variable(s) (the one(s) that must be kept constant). These can be grouped together and listed with bullet points like this: Independent variable: Length of string Dependent variable: Time taken for one complete swing Constant variables: Size of swing, mass, temperature, air resistance, force due to gravity /2 /1 /1 /2 Selects and names appropriate apparatus and materials needed. For glassware, all sizes must be listed. For chemicals, all concentrations/masses must be listed. You must also list the form of the chemical (for example if it is magnesium powder or magnesium strip). These should be listed in bullet point form. You can also show the apparatus that you intend to use using a labeled diagram. Remember to put a title on this. /2 Designs a method that allows for the effective control of the variables. This is the method of the experiment. What will you do first and second and so on. This should be done using bullet points. Be sure to add a sentence noting that when the independent variable is changed, all other variables are held constant. /2 Designs a method that allows for the collection of sufficient relevant data. To gain full marks for this criterion you need to state that three trials will be done and the results averaged to give the final number for the measurement. Repeats are two or more measurements of the dependent variable, with the same level of the independent variable. Repeats help you to assess whether your results are reliable or not. - 13 - /2 Data Collection and Processing (DCP) 1 – Collect and organise raw data Total: /18 Record all raw data (qualitative and/or quantitative) Usually the data that you collect will be quantitative - measurements with the correct units. You should record these measurements as accurately as possible during your experiment. This is usually done manually using a results table on paper. If working with a partner, do not rely on them to record the results - do it yourself. After the experiment, you should draw up a clear and accurate results table. Show every result that you obtained, not just the mean results. Repeat results should be numbered. The column headings on results table should show both the quantity being measured with the correct units. /2 Sometimes your data will be qualitative - drawings of structures, colour changes or other observations. Drawings should be large, with sharp lines and labels or annotation to interpret the structures shown. Measure the size of the specimen and the drawing and calculate the magnification. Remember to include a title for both drawings and results tables. Presents raw data clearly. /2 This means that data is collected in a table and the table is easy to read. Uses correct headings, units and significant figures. /2 2 – Processing raw data Makes the correct calculations on the raw data. The results that are collected during an experiment are called raw data. It is usually necessary to process this raw data in some way. This might involve calculating mean results, or performing a statistical test on the data. It might involve drawing a graph or displaying the data in some other way. If you are drawing a graph, remember to put the independent variable on the x~axis and the dependent variable on the y-axis. Join the points with a curve or straight lines, depending on whether you know where intervening points would have been or not. Check that you have labelled both of the axes with the quantity and the units, for example, mass (grams). If your raw data consisted of drawings, you can process them by constructing a diagram to show significant features of the structure. /2 Pays attention to units, significant figures and decimal places in final answer. /2 Extracts relevant data from the graph if drawn (intercept, gradient etc.). /2 This must be indicated on the graph. 3 – Presenting processed data Presents the processed data appropriately (correct choice of graph, bar chart etc.). Chooses an appropriate scale and plots points/displays processed data correctly/adds trendline. Uses correct labels, units and line of best fit drawn (if graph chosen). - 14 - /2 /2 /2 Conclusion and Evaluation (CE) 1 – Concluding Total: States a valid conclusion which relates to the initial problem or hypothesis What trends are shown by the data? What is the explanation for the observed differences or relationships? Identifies trend and patterns in the results How does the data compare with data from similar experiments in textbooks or scientific journals? Explains the results scientifically using relevant secondary sources. /18 /2 /2 What conclusions can be drawn from the investigation? (if any!) As in Design (aspect 1 – see above) you should use a reference here giving a literature value for the result so you can calculate the percentage error. 2 – Evaluating Comments on the reliability and accuracy of the results obtained This section should be listed under the title: “Evaluation”) /2 /2 You need to address the points on the left: Consider the following: Identifies weaknesses and errors in the procedure Are there any results that did not fit in with the rest? These are called anomalous results. /2 Were there any errors made during the experiment that explain the anomalous results? Distinguish between systematic and random error. Your main focus should be on systematic errors (these are errors that are consistently the same e.g. the balance always reads 0.6g too low). For systematic error, indicate direction and give an estimate for the magnitude of effect on final result, where possible. Identifies anomalous results and tries to explain them 3– Improvements Suggests improvements related to stated areas of weakness Proposes further improvements to increase reliability of results Suggests further work that would give more evidence for the conclusion or extend the investigation. How successfully did the method used in the experiment generate reliable results? You can often decide whether results are reliable or not by how close repeats are to each other. - 15 - What were the main weaknesses in the investigation? What could be done to make genuine improvements to the investigation, if it was done again? /2 /2 /2 /2 Manipulative (MS) 1 – Technique 2 – Instructions 3 – Teamwork Total: Is competent in the use of the technique(s) and the equipment, and pays attention to safety issues. Follows the instructions accurately Is motivated and completes the experiment & collaborates with others, recognising their needs, in order to complete the task. If you have done enough practical work in Science, your manipulative skills should be excellent. You probably will not need the following reminders! • • • - 16 - Study instructions carefully before starting work so that you know what you are doing. Be sensible about asking for help from your teacher. Try to work out what to do yourself. Use your own initiative to decide how to modify a procedure yourself when necessary. But if you have not been given full enough instructions or are worried about the safety of the procedure, ask for help.Make sure that you know about any potential risks in the procedure that you are following. Work in a careful and systematic way - arrange your apparatus tidily and do not waste time, but work without rushing. /6 /2 /2 /2 Sir Charles Darwin “I am turned into a sort of machine for observing facts and grinding out conclusions.” - 17 - 17th December 2002 Lisa Simpson IGCSE Example Lab 1 Pendulum Investigation Experiment Title – Investigate how the length of a simple pendulum affects the time for a complete swing. Design (D) Research Question – How does the time taken for one complete swing vary with the length of string for a free swinging pendulum? Hypothesis – As they string increases in length the time taken for one complete swing will increase. The diagram shows the arcs through which two pendulums swing. The longer one is twice the length of the shorter one. The shorter pendulum is always at a steeper angle than the longer arc, and always above it. The shorter pendulum has the most gravitational potential energy at the top of the swing because it is higher. This means the kinetic energy and hence speed through the centre will also be greater than for the red pendulum. From previous experiments I know that for trolleys running freely down a ramp that the bigger the angle of the ramp the bigger the acceleration of the trolley. This same principle can be applied to the falling pendulums. The steeper the arc the bigger the acceleration of the pendulum will be. A bigger acceleration means a shorter time for each swing. Unlike a ramp the arc of swing is not a straight line. The arc has the steepest gradient at the top and is flat when it reaches the middle. The acceleration of the bob will thus decrease from a maximum at the top of the swing to zero at the centre. Theory – When the pendulum is at the top of its swing it is momentarily stationary. It has zero kinetic energy and maximum gravitational potential energy. As the pendulum falls the potential energy is transferred to kinetic energy. The speed increases as the pendulum falls and reaches a maximum at the bottom of the swing. Here the speed and kinetic energy are a maximum, and the potential energy is a minimum. As the pendulum rises the kinetic energy is transferred back to potential energy. The speed of the pendulum decreases and falls to zero as it reaches the top of its swing, with the potential energy a maximum again. A small amount of energy is lost due to air resistance as the pendulum swings. This means each swing is slightly smaller than the one before. Variables Independent Variable: Length of string Dependant Variable: Time of swing Control Variables: Size of swing, mass attached to string, air resistance, gravity Method 1. Set up the experiment as shown in the diagram below – use a plasticine bob of mass 25g 2. Start with string of length 20 cm 3. Pull pendulum slightly to the side (about 10 cm) and release 4. Time 20 complete oscillations and enter into results table 5. Increase length of string by 20 cm 6. Continue until string is 160 cm long Clamp Equipment List 1. Cotton 2. Plasticine 3. Metre rulers 4. Digital stopwatch 5. Retort stand and clamp String Bob - 18 - Table Data Collection and Processing (DCP) Length √Length Number Time (cm) (√cm) of swings for N swings (s) N Average time Average time N swings 1 swing (s) 5.0 2.24 50 22.84 22.91 22.88 22.88 0.46 10.0 3.16 50 32.16 32 32.19 32.20 0.64 20.0 4.47 20 18.10 18.06 18.03 18.06 0.90 40.0 6.32 20 25.40 25.47 25.34 25.40 1.27 60.0 7.75 20 31.09 31.08 31.06 31.08 1.55 80.0 8.94 20 35.94 35.90 35.91 35.92 1.80 100.0 10 20 40.12 40.08 40.09 40.10 2.00 120.0 10.95 20 43.97 43.97 43.90 43.95 2.20 140.0 11.83 20 47.31 47.37 47.44 47.37 2.37 160.0 12.65 20 50.72 50.75 50.75 50.74 2.54 The graph on the left shows that the time taken for each swing increases as the length increases but the relationship is not linear. The rate of increase of time per swing decreases as the length increases. The graph on the right shows the linear relationship between the time taken for each swing and the square-root of the pendulum length. The equation for a straight line through the origin is; y = mx The gradient m measured from the graph = 2.5÷12.5 = 0.20 If T is the time for one swing in seconds, and L is the length in centimetres, the equation for the line can be written as; T = 0.20√L - 19 - Conclusion and Evaluation (CE) Concluding – My hypothesis was correct in that the time taken for one complete swing is directly proportional to the length of the string. However, my results show that it is directly proportional to the square root of the length of the string. All the points for the graph on the right lie on a straight line so the conclusion is very reliable over this range. It seems likely that the same trend would continue if the string was made longer or shorter. NO REFERENCE GIVEN Evaluating – The results seem to be very reliable over the range of lengths investigated. This is shown by the fact that all points on the graph on the right fit exactly on a straight line. There are no anomalous results or anomalies to be seen in the trend of the graph. Sources of weaknesses and error in the procedure include: 1. 2. I had to estimate where the centre of the bob was when measuring the length of the string. I estimated the uncertainty in this as 1 mm. Also, when measuring the length beyond 1 metre, it must be done in two parts using metre rulers. Human reaction time: This come in two parts: a) When using the stop watch: This source of error roughly cancels out as the human reaction time to start and stop the watch as the same event is being observed, and reacted to in the same way, each time. Errors are produced by any variability in the reaction time of the individual which could be affected by many things. Taking more time measurements may give a slightly more accurate average for each length, but not by much. b) When deciding when one full swing has taken place: This was done by my judgement. However, several improvements could be made for improving the accuracy of this measurement and they are outlines in the improvements section below. Very short lengths will run into logistical issues. It could be hard to measure the number of complete swings if they happen very quickly. For very short lengths the trend may not continue. It is not possible to try lengths shorter than the diameter of the bob, for instance. Improvements – The procedure used was simple and straightforward and no difficulties were encountered. Several improvements would add to the accuracy of the result: 1. 2. 3. 4. A longer ruler could be placed level with the point of suspension, and a set square could be placed along the flat side and just touching the bottom of the pendulum. This distance could then be measured more accurately than trying to guess where the middle of the bob is. The diameter of the bob could be accurately measured with some vernier callipers so that the true length of the pendulum could then be calculated. The thread used was quite stretchy. If the investigation was repeated I would replace it with something more rigid, such as extra strong button thread. A greater range of lengths both longer and shorter than what I measured should be conducted. At very long lengths, a stronger string and a bob with more mass maybe needed to counter air resistance. Shorter lengths would reach a limit would be reached where the pendulum moves too quickly to be accurately counted. The use of an electronic Pasco sensor such as a light gate could help measure very fast periods accurately. Also, a very high speed digital video camera that could accurately record the position of the bob and the elapsed time. 5. More repeats could be taken but I don't think this would add much to the accuracy of the conclusions. 6. The investigation could be extended by investigating the relationship between period and length of string for circular swings. - 20 - - 21 - - 22 - - 23 - - 24 - - 25 - - 26 - - 27 - - 28 - - 29 - - 30 - - 31 - - 32 - - 33 - - 34 - - 35 - - 36 - You must know these chemical tests off by heart! - 37 - - 38 - Studying Effectively You need to organize yourself to study effectively. To manage time effectively you should set yourself a schedule of study in order to organize and prioritize your studies in the context of competing activities of sport, family, etc. Follow up on the priorities you have set for yourself, and don't let others or other interests, distract you from your goals. One way to help with this is to create a study schedule. An example of a study schedule is this: Preparing for Tests and Exams Create study checklists Identify all of the material that you will be tested on-- list notes, formulas, ideas, and text assignments you are accountable for. This checklist will enable you to break your studying into organized, manageable chunks, which should allow for a comprehensive review plan with minimal anxiety Create summary notes and "maps" Briefly map out the important ideas of the course and the relationships of these ideas. Summary notes should display lists and hierarchies of ideas. Creativity and a visual framework will help you recall these ideas. Create flashcards Flashcards are useful for definitions, formulas, or lists that you need to have memorized--put topics on one side of the card, answers on the other. Flashcards will enable you to test your ability to not only recognize important information, but also your ability to retrieve information from scratch - 39 - Glossary WORD MEANING abdomen part of the body between the chest and hip, containing organs like the stomach, liver and intestines. absorb soaking something up from the surroundings. A sponge absorbs water. acid a substance that turns litmus red . Has a pH of less than 7. acid rain rain containing sulphuric acid and nitric acid. Acid rain has a pH of less than 5.6. adapted when a cell or organism has certain features to help it to do a particular job. When the shape of a cell helps it to do its job it is said to be 'adapted' to its job or function. addictive a drug that causes the user to become dependent on it. aerobic the main respiration reaction in cells. It uses oxygen from the air and glucose from respiration food to release energy . The waste products are carbon dioxide and water. alcohol one of a group of chemicals with similar properties. Ethanol is the scientific name for the chemical found in alcoholic drinks. alcoholic someone who is dependant on alcoholic drinks. alkali a substance that turns litmus blue. an alkali has a pH of more than 7. Another name for a base that dissolves in water. alkaline with a pH of more than 7. alternating current an electrical current that flows one way then another. Generators and the mains supply alternating current. alveoli tiny pockets in the lungs at the ends of the bronchioles, where oxygen diffuses into the blood and carbon dioxide diffuses out. amino acid the molecules that proteins are made of. ammeter a piece of equipment used to measure electrical current. amp(A) the unit for measuring electrical current. anaerobe a living thing which survives without oxygen. anaerobic respiration respiration without oxygen, which takes place when cells cannot get enough oxygen to meet their energy needs by aerobic respiration. anode positive electrode in electrolysis. antibodies proteins that destroy particular microbes. They are made by white blood cells. antitoxins chemicals that destroy toxins. They are made by white blood cells. anus the opening at the end of the gut. aqueous solution a solution of something in water. - 40 - armature the turning part of a motor or generator. artery a blood vessel that carries blood away from the heart. atom the smallest particle of an element. it has no overall electric charge. atomic mass the relative mass of an atom. Roughly how many times heavier it is than an atom of hydrogen. atomic number the number of protons in an element's atom. atrium the upper space on each side of the heart. it receives blood from the veins. attract pulling something closer. auxin plant growth hormone. it is found in the tips and shoots and the roots. bacterium a single celled microbe without a cell nucleus can cause a disease. barrage large dam across a river to control the flow of the tides. base a substance with reacts with an acid to form a salt. some bases are alkalis. battery two or more electrical cells used together. bauxite a rock containing aluminium. biodegradable something which will decay naturally bronchi pair of large air tubes from the trachea to the lungs. bronchioles small air tubes that branch out from the bronchi inside the lungs. capillaries tiny blood vessels that link arteries and veins. carbon monoxide very poisonous colourless gas. it stops red blood cells carrying enough oxygen around the body. catalyst speeding up a chemical reaction. cellulose the substance that plant cell walls are made from. cholesterol a fatty substance found in some foods. it can clog up arteries and lead to heart disease. chromosome a thread like strand found in the nucleus of a cell. made from DNA and contain instructions for a living thing. cilia small hairs on the surface of some cells. circuit breaker an electromagnetic switch the breaks the circuit if the current gets too big. convection current a flow of liquid or gas caused by part of it being heated or cooled more than the rest. digestive system organ system used to break down food and change it into a form the body can use. diabetes when the pancreas produces too little insulin. discharge removing electrical charge from something. - 41 - discontinuous deposition when the sedimentary rocks found at a place were not laid down continuously, but at different times. electrolyte a liquid that conducts electricity emulsion a mixture of tiny droplets of one liquid blended throughout another liquid. epithelium tissue layer of cells that cover different body surfaces, ethene a hydrocarbon gas. faeces the undigested and unabsorbed remains of food. fractional distillation a process for separating a mixture fruit an organ that carries the seeds of flowering plants. can be fleshly or dry. fuse rating the maximum current that a fuse will conduct without melting. gastric juice digestive juice made by glands in the stomach lining. it is very acidic and contains enzymes to break down proteins. global warming gradual heating of the earth's atmosphere. it is caused by the 'greenhouse effect'. gravitational potential energy the kind of energy stored by anything that can fall to the ground. gullet the tube from the back of the mouth to the stomach. muscles in it contract and relax to push the food along. heat conductor a material that lets heat energy flow through it easily. hydroxide a compound containing immune protected against catching a disease because the body has already made the right antibodies. insoluble something that will not dissolve kidneys a pair of organs used to clean the bolld. they remove the urea in the blood and make it into urine. kinetic energy the kind of energy in moving things lactic acid the waste product of anaerobic respiration. if it builds up in the muscles it makes them ache. litmus a simple kind of indicator. it is red in acids and blue in alkalis. metal oxide a compound of a metal and oxygen. metamorphic a rock that has been changed by great heat or pressure(e.g. marbel) native when somehting occurs in nature as the element itself, not as a compound newton the unit for force. ohm (W) the unit for measuring electrical resistance oil a fossil fuel made from the remains of animals optic nerve the nerve that carries messages from the retina to the brain - 42 - ore a rock that contains useful minerals organ a group of different tissues working together organ system a collection of organs working together organism any living thing. An organism must do all seven of the 'life processes'. osmosis when water flows through a semi-permeable membrane so that the concentration on either side becomes more equal overload a fuse is overloaded if the current gets too big and melts it oxide a compound of an element and oxygen oxidise, oxidation adding oxygen to a chemical, removing electrons, removing hydrogen oxygen a colourless gas that makes up about 20% of the air. It is produced by photosynthesis and used up in respiration oxygen debt the amount of oxygen needed to remove the lactic acid left from anerobic respiration palisade cell a cell found in leaves, which contains many chloroplasts palisade tissue many palisade cells grouped together pancreas the organ that secretes digestive juices and hormones parallel (electricity) when the current in an electric circuit can flow along different routes period horizontal row of elements in the Periodic Table Periodic Table chart with the chemical elements arranged in order of atomic number. Elements with similar properties appear in the same coloumn permanent magnet something that attracts iron all the time pholem tissue living cells grouped together to carry dissolved food substances from the leaves to other parts of the plant photosynthesis process that plants use to make their own food. It neds light to work. Carbon dioxide and water are used up. A sugar called glucose , and oxygen are produced photosynthesise making food by photosynthesis plant hormone chemicals that controls the way a plant grows plant organ group of different plant tissues working together to do an important job plasma the liquid part of the blood, which is mainly water. It carries many substances around the body (eg hormones, waste carbon dioxide and urea, nutrients platelets tiny pieces of cell in the blood that release chemicals to help blood clot. They come from cells in bone marrow plutonium a fuel used in nuclear power stations pole the ends of a magnet, where its effects are strongest polyethene the chemical name for polythene. Its a polymer of ethane - 43 - polymer a long molecule made from thousands of smaller ones (monomers). Plastics are polymers potential difference another name for voltage potential energy the scientific name for 'stored' energy power how quickly something transfers energy power line overhead or underground cables that carry electricity process sorting out information product a substance formed by a chemical reaction property a way that a substance behaves proportional two quantities are proportional to each other if doubling one of them makes the other one double too protein coat outer coating of a virus proteins important substances used up for growth and repair proton tiny positevely charged particle in an atoms nucleus pupil gap in the middle of the isis of the eye pure a single substance, not mixed with anything else pus the remains after many microbes have been ingested by white blood cells at a spot or cut pylon a tall tower holding up overhead power cables quarry a place where useful rocks are dug out of the ground quicklime substance made by heating limestone. Its chemical name is calcium oxide radiate giving off waves of energy. A candle radiates light and heat energy radiation the way heat travels as waves of energy through space or transparent materials. This can also refer to alpha, beta, gamma radiation radioactive waste dangerous waste from nuclear power stations rate the speed of a chemical reaction raw materials another term for a reactant reactant a substance used up in a chemical reaction reactive a substance that is likely t react reactivity Series list of metals and non metals arranged in order of how reactive they are receptors cells that detect change in the body or its surroundings rectum the last part of the large intestine, leading to the anus. It stores Faeces red blood cells the cells that give blood its colour. They contain haemoglobin, which carries oxygen around the body - 44 - reduce/reduction taking oxygen away from/adding hydrogen to a compound in a chemical reaction. Also defined as: taking away electrons (oxidation) adding electrons (reduction). reducing agent a chemical that will reduce other substances refinery place where the chamicals in crude oil are seperated and purified reflect bouncing something backfrom a surface reflex action an automatic response to a stimulus, often to protect the body from harm relax when a muscle relaxes after contracting it goes back to its original shape relay an electromagnetic switch for turning large currents on safely renewable an energy source that can be replaced or used again and again, and will never run out (eg solar power) repel pushing something away resistance how difficult it is for an electrical current to flow through something resistor an electrical component that decreases the current in a circuit respiration chemical reaction inside cells to release energy from glucose response how the body reacts to a stimulus that has been detected (eg opening up the pupil in dim light retina the back of the eye. It contains receptors that are sensitive to light ripple mark patterns left behind in sedimentary rock from the time when the sediment was under water rock cycle all the processes which form rocks, linked together root plant organ used to hold the plant in the gound and take water and mineral salts out of the soil root hair cell cell found in the roots. It has a large surface area to help the cell absorbs water quickly root hair tissue many root hair cells grouped together rusting corrosion of iron by water and oxygen sacrificial protection allowing a piece of reactive metal to corrode so that an object made of a less reactive metal does not saliva secretion from the salivary glands. It contains enzymes to break down starch, and mucus to help food pass smoothly down the gullet salt a compound formed when an acid eacts with a base scab hard protective covering over a cut that forms when a blood clot dries sclera protective outer layer of the eye secretion useful substance (eg tears, saliva, hormones) made by gland cells sediment tiny particles that settle to the bottom of a liquid - 45 - sedimentary rock formed by the compression and cementing of material that has settled at the bottom of the sea seed grows into a new plant. Made by flowering plants and conifers semi-permeable a membrane that will let small particles, like water, through it but not large ones sensory neurone a nerve cell that carries messages from a receptor to the brain or spinal cord series electrical components connected 'in line' so that all of an electrical current flows through each one, one after another sex organ the stamea (male) and carpel (female) in a flower. They make the male and female sex cells slaked lime a base made from limestone. its chemical name is calcium hydroxide small intestine the organ used to digest and absorb food solar cell a kind of batterythat generates electricity using energy from the Sun soluble something that can dissolve in a liquid (eg salt is soluble in water) solution a solute (substance) dissolved in a solvent (liquid, usually water) solvent a chemical used to dissolve something solvent abuse breathing in solvent fumes on purpose sound energy the kind of energy given out by something that makes a noise South Pole/South-seeking pole the end of a freely suspended bar magnet that points south spinal cord large bundle of nerve cells that carry messages to and from the brain. It runs down the back, inside the spine stain dye used to colour parts of a cell to make them easier to see stainless steel mixture of iron with chroniun, corbon and other elements. It does not rust starch carbohydrate that plants use as a store of food stem leaves plant organ used to support a plant and take water and mineral salts to the stimulus (plural stimuli) change within the body or in its surroundings that receptor detects (senses) stomach organ used to help break down food. It secretes digestive juices stomata (singular stoma) small holes on the underside of leaves which let gases into and out of the leaves part of a plant where a food substance can be stored storage organ sucrose the chemical name for the sugar used in cooking. Some plants (eg sugar beet) make it from glucose sugars group of carbohydrates that dissolve in water and taste sweet sulphur dioxide a gas that is produced in small quantities when fossil fuels are burnt. It is a cause of acid rain surface area the total area of all surfaces of a shape - 46 - suspensory ligaments part of the eye. They work with the ciliary muscle to hodls the lens in place and changes its shape symbol equation shorthand way of showing what happens in a chemical reaction using symbols symptom sign that the body has a disease target cell a cell that is affected by a hormone target organ an organ that is affected by a hormone taste bud receptor on the tounge which is sensative to flavours tactonic plate a section of the Earths lithosphere tempory magnet something that can be made to attract iron when needed terminal where an electrical connection in made thermal decomposition breaking down a compound by heating it thermal energy another name for heat energy thorax the chest, containg the lungs and heart tides twice-daily rising and falling of sea level, caused by the pull of the Moon tissue a group of the same cells all doing the same job tabacco the dried leaves of the tabacco plant toxin poisonous substance made by a living thing trachea the main air tube to the lungs transfer the word used for heat or energy moving from place to place transformer piece of equipment that increases or decreases voltage. A step-up transformer increses voltage and a step-down transformer decreases voltage transmit getting electricity from one place to another transpiration loss of water from plants leaves transpiration stream the flow of water up through a plants roots and stem to its leaves transport (in earth science) when eroded fragments are moved away from their 'parent rock' by wind or water turbine a machine that is turned by a moving fluid unit a unit for measuring the amount of electrical energy transfered. 1 unit is the same as 1 kilowatt-hour universal indicator a special mixture of indicators. It gives a different colour depending on how weak or strong an acid or an alkali is unlike opposite charges os magnet poles uranium a fuel used in nuclear power stations urea waste product from the breakdown of unwanted amino acids by the liver urinate getting rid of urine when you go to the toilet - 47 - urine solution of the bodys waste products, which are removed from the blood by the kidneys vaccination being given an injection of a vaccine to help the body protect itself against disease vaccine weak or dead disease causing microbes put into the body on purpose. White blood cells make the right antibodies and the person becomes immune valve part of a vein or the heart that stops the blood in it from flowing the wrong way vapour another name for gas vegetable any plant food that is not a fruit xylem tissue xylem cells grouped together in tubes that carry water and mineral salts up from the plants roots to the leaves - 48 - - 49 - - 50 -