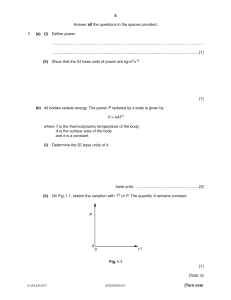
Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 Contents lists available at ScienceDirect Bioorganic & Medicinal Chemistry Letters journal homepage: www.elsevier.com/locate/bmcl Digest Breakthroughs in neuroactive steroid drug discovery Maria-Jesus Blanco ⇑, Daniel La, Quinn Coughlin, Caitlin A. Newman, Andrew M. Griffin, Boyd L. Harrison, Francesco G. Salituro Sage Therapeutics, Inc., 215 First Street, Cambridge, MA 02142, USA a r t i c l e i n f o Article history: Received 7 October 2017 Revised 26 November 2017 Accepted 27 November 2017 Available online 2 December 2017 Keywords: Neurosteroid NAS GABAA NMDA Synaptic Extrasynaptic Clinical candidate a b s t r a c t Endogenous and synthetic neuroactive steroids (NASs) or neurosteroids are effective modulators of multiple signaling pathways including receptors for the c-aminobutyric acid A (GABAA) and glutamate, in particular N-methyl-D-aspartate (NMDA). These receptors are the major inhibitory and excitatory neurotransmitters in the central nervous system (CNS), and there is growing evidence suggesting that dysregulation of neurosteroid production plays a role in numerous neurological disorders. The significant unmet medical need for treatment of CNS disorders has increased the interest for these types of compounds. In this review, we highlight recent progress in the clinical development of NAS drug candidates, in addition to preclinical breakthroughs in the identification of novel NASs, mainly for GABAA and NMDA receptor modulation. Ó 2017 Elsevier Ltd. All rights reserved. Neuroactive steroids (NASs) or neurosteroids are among the most potent and effective modulators of neuronal excitability. The term ‘‘neurosteroid” was first mentioned by Etienne Baulieu in 19800 s and initially referred to endogenous steroids synthesized in the brain and central nervous system (CNS) from cholesterol.1 Neurosteroids have been shown to impact CNS function primarily through allosteric modulation of the GABA (c-aminobutyric acid)A receptor (GABAAR) or the N-methyl-D-aspartic acid class of glutamate receptors (NMDAR). However, at high concentrations neurosteroids have been shown to act on other receptor systems like nicotinic acetylcholine, serotonin 5-HT3, and sigma1 receptors. The term neurosteroid has been expanded to include synthetic and naturally-derived analogs that have CNS actions similar to endogenous neurosteroids. Increasing evidence2,3 indicates that dysregulation of neurosteroid production plays a role in the pathophysiology of stress and stress-related psychiatric disorders, including mood and anxiety disorders. In addition to agonist or antagonist modes of action at different receptors, these receptors could be either positively (PAM) or negatively (NAM) modulated by NAS compounds at allosteric sites. For example, allopregnanolone (1) is a PAM at the GABAAR (Fig. 1). Other endogenous NASs, such as 24(S) hydroxycholesterol (2) are PAMs at NMDA receptors.4 Such mechanisms are providing novel approaches to treat CNS disorders, and the steroid field is ⇑ Corresponding author. E-mail address: Maria-Jesus.Blanco@sagerx.com (M.-J. Blanco). https://doi.org/10.1016/j.bmcl.2017.11.043 0960-894X/Ó 2017 Elsevier Ltd. All rights reserved. showing a significant resurgence with multiple compounds advancing to clinical studies. Clinical trials currently underway are assessing the efficacy of various NASs for the treatment of diverse CNS disorders such as epilepsy,5 super refractory status epilepticus (SRSE), Fragile X, traumatic brain injury and Alzheimer’s disease.3 In this BOMCL Digest, we will provide a brief perspective on recently disclosed GABAAR and NMDAR NASs that have either advanced to clinical studies or have been described preclinically within the last 3–5 years. Clinical NASs. Ten NAS compounds have reached clinical development status since the 19700 s (Table 1), however many have since been withdrawn. For example, an intravenous anesthetic combining two NASs, alphaxolone (3) and alphadolone in a cremophor vehicle was withdrawn from the market in 1984 due to issues with anaphylaxis. Later in 2001, it was determined that Cremophor EL was responsible for these anaphylactic reactions in humans.7 Currently, alphaxolone alone is under additional human studies in a sulfobutyl ether-b-cyclodextrin formulation (‘‘Phaxan”) as an intravenous anesthetic.8 Minaxolone (CCI-12923, 4), a GABAAR PAM, was developed as a water-soluble anesthetic NAS. It was withdrawn before registration due to toxicity observed in long-term studies in rats.9 Early emphasis on water-soluble NASs led to the discovery of ORG20599 (5), a potent GABAAR PAM. Unacceptable clinical profile, however led to withdrawal from further clinical development.10 Marinus Pharmaceuticals has been developing ganaxolone (GX, 6), a 3b methyl derivative of allopregnanolone for focal-onset 62 M.-J. Blanco et al. / Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 Fig. 1. Structures of Allopregnanolone (1) and 24 (S) hydroxycholesterol (2). Generic steroid nomenclature used in this manuscript. aCarbon numbering and ring designations of the steroid core.6 Substituents above the plane of the paper are described as b and are shown as a solid line; those below the plane are described as a and are shown by a broken line. Carbons at positions 4, 5 and/or 6 may be saturated or unsaturated. seizures in adults and in children with epilepsy. Focal-onset seizures are manifestations of abnormal epileptic firing of brain cells. It is estimated that 65 million people worldwide are living with some form of epilepsy.24 A disclosure in 2016 announced the discontinuation of phase 3 clinical studies for adult focal onset seizures and advancing 6 in status epilepticus and pediatric orphan indications.11 GABAAR antagonist sepranolone12 (7, Table 1) is currently in clinical studies for the treatment of premenstrual dysphoric disorder (PMDD). This disease is a severe, debilitating form of premenstrual distress comprising emotional, physical symptoms and functional impairment. PMDD affects 3–8% of women in fertile ages.25 A phase 2b trial in PMDD patients was expected to begin in the second half of 2017 after an initial phase 1/2 study demonstrated a statistically significant difference between active treatment and placebo. Bruschettini SRL is developing tauroursodeoxycholic acid [8, TUDCA, the taurine conjugate of ursodeoxycholic acid (UDCA)] for the treatment of amyotrophic lateral sclerosis (ALS).13 ALS is a progressive CNS disease where nerve cells in the brain and spinal cord that control voluntary movement gradually deteriorate, causing loss of muscle function and paralysis. Several studies have demonstrated that TUDCA imparts anti-apoptotic activity in a number of neurodegenerative diseases, including ALS, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease.26 It has been indicated that UDCA modifies the function of the bile salt export pump (BSEP, ABCB11) however underlying mechanisms remain unknown. On February 27th 2017, orphan designation was granted by the European Commission for TUDCA for the treatment of ALS. The Shanghai Innovative Research Center of Traditional Chinese Medicine (SIRC-TCM) is developing S-111 (9, Yuxintine, or 20(S)protopanaxadiol, Table 1), an active ginseng intestinal metabolite and an inhibitor of serotonin and norepinephrine uptake, for the treatment of depression, including major depressive disorder (MDD). MDD is a widely distributed medical condition that includes abnormalities of mood, appetite, sleep, cognition and psychomotor activity.27 Preclinical antidepressant-like activity of orally administered S-111 was measured in various animal models of depression and demonstrated antidepressant-like activity with similar potency to fluoxetine.28 The latest report in 2015, indicated the drug was in phase 2 development.14,15 Sage Therapeutics has recently disclosed phase 2 results for its first generation NAS, SAGE-547 (10, Brexanolone).29 A parenteral, continuous infusion formulation of SAGE-547, was in phase 3 clinical trials for the treatment of SRSE, a life-threatening condition in which the brain is in a state of persistent seizure that fails to respond to standard treatments.18,30,31 The study did not meet the primary endpoint,32 comparing success in weaning of thirdline agents and resolution of potentially life-threatening status epilepticus with brexanolone vs. placebo when added to standard-of-care. In addition, SAGE-547 has completed a phase 2 clinical trial in severe post-partum depression (PPD)29,33 and an exploratory study in essential tremor (ET).34 Simultaneous with the development program in SRSE, SAGE-547 was studied in a phase 3 program in moderate and severe post-partum depression.29 There is considerable preclinical research supporting the potential for GABAAR modulation imparting benefits in a number of mood disorders. For post-partum depression in particular, there is evidence sustaining the potential utility of NASs, such as allopregnanolone, in depressive mood disorders through modulation of synaptic and extrasynaptic GABAARs.35 This disease, with no approved drugs to date, is estimated to affect between 10 and 20% of women in the United States after childbirth.36 In early November 2017, Sage Therapeutics announced positive top-line results for phase 3 studies in moderate and severe post-partum depression. Brexanolone achieved the primary endpoint in both trials with a mean reduction from baseline in the Hamilton Rating Scale for Depression (HAM-D) total score compared to placebo at 60 h.37 SAGE-547 is a potent GABAAR PAM, active at both synaptic and extrasynaptic GABAARs, and is ideally suited for parenteral administration, due to its high intrinsic clearance and low volume of distribution, yielding a fast on/fast off pharmacokinetic profile.38 In searching for next generation NASs, Sage Therapeutics has developed a molecule with robust pharmacological PAM activity at GABAAR but with low intrinsic clearance, high oral bioavailability and potential for once daily dosing aiming to reach larger populations of patients. To this end, Sage Therapeutics reported the discovery of SAGE-217 (11, Table 1),38,39 a clinical candidate which has now completed single and multiple ascending doses (SAD, MAD) phase 1 clinical trials and has progressed into phase 2 clinical trials for the treatment of GABAARs mediated movement disorders such as essential tremor (ET) and Parkinson’s disease as well as in mood disorders such as PPD and MDD. In April 2017, Sage Therapeutics announced the advancement of SAGE-718 (12, Table 1) into phase 1 clinical studies.23 SAGE-718 is a novel, oral, first-in-class oxysterol-based PAM of the NMDAR. Positive modulation of NMDARs has potential benefit in the treatment of a range of neurological disorders associated with a variety of cognitive, neurological and behavioral symptoms. SAGE-718 also has potential in the treatment of CNS disorders associated with a high prevalence of anti-NMDA antibodies or reduced levels of endogenous 24(S)-hydroxycholesterol (2). In preclinical studies, SAGE-718 improved social behavior in an animal model of NMDA hypofunction, and ameliorated both behavioral and electrophysiological deficits in a model of compromised cholesterol regulation.23 In general, development of NASs faces several challenges as seen from previous examples. Many of the issues are related to formulation40 as those compounds tend to have physicochemical properties outside of the traditional small molecule drug-like properties (Table 1). Attempts were made to reduce lipophilicity of the compounds and increase aqueous solubility (4, 5), however the compounds led to unacceptable margins of safety. It is important to highlight that the receptors are membrane bound. Chisari41 postulated that NASs might require a membranous route of access to 63 M.-J. Blanco et al. / Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 Table 1 Progression of NASs in clinical development. a Compd number Compd name 3 Structure Company/ organization Current Status Ref. Physicochemical properties (MW/ cLogP/PSA)a Alphaxolone & alphadolone (Althesin) Alphaxolone (Phaxan) Glaxo Research Group Ltd (Althesin) Drawbridge Pharmaceuticals (Phaxan). Withdrawn from the market (1984). Alfaxolone still used for veterinary medicine. Alphaxalone alone (Phaxan) is currently in human studies as anesthetic formulated in sulphobutyl-ether bcyclodextrin. 7,8 332.5/3.7/54.4 4 Minaxolone (CCI-12923) Glaxo Research Group Ltd Withdrawn before registration (1979) 9 405.6/3.1/49.8 5 ORG-20599 Organon International Halted further clinical studies (2001) 10 438.1/4.3/49.8 6 Ganaxolone (GX) Marinus Pharmaceuticals Phase 3 for adult focal-onset seizures discontinued (6/2016). Phase 2 studies ongoing for epilepsy indications (status epilepticus and pediatric orphan indications). 11 332.5/5.0/37.3 7 Sepranolone Swedish Asarina Pharma Phase 2, premenstrual syndrome (GABAA Antagonist); iv 12 318.5/4.5/37.3 8 Tauroursodeoxycholic acid Bruschettini SRL Phase 2, Motor neuron disease (ALS); oral 13 499.7/2.1/123.9 9 S-111 (Yuxintine) Shanghai Innovative Research Center of Traditional Chinese Medicine Phase 2, Major depressive disorder (MDD); oral 14,15 460.4/6.8/60.7 10 SAGE-547 (Brexanolone) Sage Therapeutics Achieved primary endpoints for two Phase 3 studies in postpartum depression (PPD, 11/ 2017); Phase 2 for Essential tremor (ET). Discontinued Phase 3 for super refractory status epilepticus (SRSE, 9/2017). 16–18 318.5/4.5/37.3 11 SAGE-217 Sage Therapeutics Phase 2, Severe PPD, MDD, ET, Parkinson disease 19–22 409.6/4.3/76.7 12 SAGE-718 Sage Therapeutics Phase 1 (4/2017) 23 Undisclosed Physico-chemical properties calculated using ChemDraw 16.0 software. PerkinElmer Informatics Inc. transmembrane-domain binding sites within GABAAR. As more compounds move into development, there is an opportunity to understand the translation from preclinical to clinical studies including target engagement,42 dose, route of administration, duration, and safety. Preclinical Neurosteroids. Moving into the second part of the manuscript, the focus will be on summarizing preclinical reports on GABAAR and NMDAR modulators. Each section will include a brief introduction on the receptor. Preclinical GABAAR NAS PAMs. The GABAAR is a pentameric ion channel surrounding a central chloride ion-selective channel gated by GABA (Fig. 2). Functional receptors consist of five subunits selected from 19 known receptor subunits: a1–6, b1–3, c1–3, d, e, h,p, and q1–3. This specific assembly results in a complex 64 M.-J. Blanco et al. / Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 Fig. 3. Oxygen bridge NAS analogs described by Coirini et al. including IC50 inhibition values of [35S]-TBPS binding to male rat synaptosomes. Fig. 2. Schematic representation of the GABAAR. heterogeneity of GABAAR subtypes, and each subtype has a distinct physiological and pharmacological profile. Distinct receptor subtypes have a specific regional and cellular expression pattern. For example, GABAARs that contain a1–3, b1-3, and c2 subunits are mainly synaptic, whereas a4–6 and d-containing receptors are mainly peri- or extrasynaptically located. Allopregnanolone and other NASs are potent allosteric modulators of both synaptic and extrasynaptic GABAARs. Hence, NASs can enhance synaptic phasic and extrasynaptic tonic inhibition. The resulting chloride current conductance generates a form of shunting inhibition that controls network excitability, seizures, and behavior.43 Structurally, NASs have an ABCD steroid core and follows the steroid nomenclature depicted in Fig. 1. NASs have the potential to differentiate from classical GABAAR modulators in several ways. First, NASs differentiate from benzodiazepines by targeting different populations of GABAARs. NASs have been suggested to putatively bind to three or four distinct sites on the GABAAR (Fig. 2). However, benzodiazepines bind to an allosteric site distinct from Compound 1 a the GABA-binding site, at the interface of the a and c subunits. This limits their ability to potentiate synaptic GABA currents to receptor assemblies that contain a c subunit. Conversely, NASs potentiate the GABAAR by binding to residues within the a subunit and so modulate receptors independently of their subunit composition. Therefore, unlike benzodiazepines, NASs are capable of targeting extrasynaptic GABAAR that include the d subunit in addition to synaptic c-containing receptors. As such, NASs may exhibit a therapeutic advantage44 over benzodiazepines that has led to renewed medicinal chemistry efforts to explore novel analogs. For example, Coirini and colleagues45 recently reported the synthesis of allopregnanolone and pregnanolone analogs with an intramolecular oxygen bridge between A and B rings (Fig. 3). These conformationally constrained analogs were hypothesized, according to the authors, to possess a more favorable spatial arrangement for binding. The compounds (13–16) were evaluated by [35S]-TBPS binding competition assay and shown to have similar activity in this assay relative to allopregnanolone (1, IC50 = 86 nM reported). Compound 14 was further evaluated in cerebral cortex and hippocampus cultures subjected to hypoxia to evaluate neuroprotective activity. Compound 14 was shown to prevent the increase of glial fibrillary acidic protein as well as neurofilament (NF160/200) decrease in hippocampus cultures subjected to ischemia. Although no DMPK [35S]-TBPS binding competition assay IC50 (nM) 80.0 + 14.3 17 255.3 + 17.9 18 625.0 + 125.0 19 5000.0 + 100.0 IC50 records the steroid concentration producing a half-maximal inhibition of the [35S]-TBPS binding competition assay. Fig. 4. Epoxide neurosteroids reported by Kasal and coworkers. 65 M.-J. Blanco et al. / Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 Activity/property GX (6) UCI-50027 (20) 0.2 1.2 0.1 0.2 Solubility 20 mM in 45% HP CD 52 mM in 20% HP CD Rat %F No data reported 1 2 2L GABAAR EC50 (oocytes, M) 2 1 2L GABAAR EC50 (oocytes, M) b In vivo activity (mouse, po) EPM MED= 20 mg/kg Preliminary safety (Mouse) aReported 77a c b PTZ EPM MED < 0.3 mg/kg ED50 = 23 mg/kg PTZc ED50 = 6 mg/kg TI as anxiolytic (EPM) = 3.3 TI as anxiolytic (EPM) > 127 TI as anticonvulsant = 3 TI as anticonvulsant ~ 6 dosing 3 mg/kg p.o. and 1 mg/kg i.v. bEPM= elevated plus maze (EPM) model for anxiety; cPTZ = pentylenetetrazole-induced seizures assay Fig. 5. Comparison of preclinical profile of UCI-50027 (20) vs. GX (6). Fig. 7. Nor-19-pregnanolone analog SGE-516. NAS Protection against A 42 toxicity on adult neural stem cells 1 + 21 ++ 22 ++ Table 2 In vitro GABAA pharmacology and DMPK profile for SGE-516.49 Fig. 6. O-allyl neurosteroid analogs described by Karout et al.48 a1b2 c2 EC50 (nM)/Emax (%) a4b3d EC50 (nM)/Emax (%) Rat Oral% Fa Brain:plasma ratioa 125/663 240/579 27 1.8 a data or other supporting data was provided, the authors stated that these oxygen bridge analogs might offer promise in other therapeutic applications. Kasal et. al. have explored the hypothesis that a 2a,3a-epoxy ring could replace the 3a-hydroxyl group,46 synthesizing several analogs (Fig. 4). Epoxides 17 and 18 displayed somewhat weaker inhibition relative to 1, while 19 displayed significantly lower activity than 1. Additionally, the corresponding diol formed by epoxide opening, 2b,3a-dihydroxy-5a-pregnan-20-one, did not inhibit [35S]-TBPS binding. This result demonstrated that the activity of epoxide 17 is due to the compound itself and not to its hydrolytic product. Compound 17 exhibited moderate anticonvulsant and short-lasting activity when tested in motor seizures induced by pentylenetetrazol (PTZ) at 20 mg/kg. The authors highlighted that although the epoxide could be an acceptable substitution for 3a-hydroxy group, in vivo results showed that the epoxide analogs are highly metabolized. Hogenkamp47 and colleagues have studied potential novel bioisostere replacements of the 17b-acetyl side chain to improve the pharmacological profile of GX (6, Table 1). Isoxazoles are known In vivo PK parameters following iv (5 mg/kg) and oral (20 mg/kg). Brain:plasma ratios are obtained by single point at 30 min post iv. Oral bioavailability and brain:plasma ratios were calculated from mean plasma concentrations from two rats. bioisosteres for ketones, and in this case, it was postulated that an isoxazole would maintain activity at GABAARs while improving the DMPK profile of GX (6). This work led to the discovery of 3-[3a-hydroxy-3b-methyl-5a-androstan-17b-yl]-5-(hydroxymethyl)isoxazole also known as UCI-50027 (20, Fig. 5). Like 6, UCI-50027 (20) was shown to have activity as GABAA PAM when tested in Xenopus oocytes expressing a1b2c2L and a2b1c2L GABAARs (Fig. 5). While less potent than 6 in vitro, UCI-50027 is more potent in vivo both as an anxiolytic and as an anticonvulsant. The minimum effective dose (MED) for UCI-50027 is 0.3 mg/kg when administered orally in the mouse elevated plus maze (EPM) paradigm, while GX has an MED of 20 mg/kg. Similarly, UCI-50027 is more potent than GX in anticonvulsant models. The ED50 for UCI50027 is 6 mg/kg when administered orally in mouse against PTZ-induced seizures assay, while GX has an oral ED50 of 23 mg/ kg. The authors hypothesized that the subtype receptor selectivity could explain the differences in the anxiolytic profile between the 66 M.-J. Blanco et al. / Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 Table 3 Activity of SGE-516 in mouse PTZ-induced seizure assay and PK parameters.50 a Dose (mg/kg, po) % clonic seizure % tonic seizure % death Plasma concentration (ng/mL)a Brain concentration (ng/g)a vehicle 3 10 30 100 90 20 0 80 40 0 0 80 30 0 0 NA 87 703 1689 NA 87 773 1955 All samples collected 30 min after administration. Table 4 Correlation between NASs modulation of tonic current and anticonvulsant profiles in the 6-Hz seizure model in mice.57 Compound NAS-mediated potentiation of tonic currents by 1 lM GABAa 6-Hz Test ED50 (mg/kg) 1 24 25 26 6 3 4.36 2.24 0.76 3.52 3.47 1.98 4.2 7.7 >100 5.0 1.5 8.8 a Values derived from fold potentiation of mean, normalized tonic current response to 1 lM GABA. Tonic current responses were recorded from voltageclamped (65 mV) DGGCs from wild type female mice. All mean values are representative of four to eight cells per neurosteroid and concentration. two compounds (and it was not linked to brain penetration profile). Pharmacokinetic (PK) studies indicated that UCI-50027 showed larger exposures than GX when dosed orally in rats. When UCI-50027 was dosed orally at 3 mg/kg in rats, the maximum plasma levels were 200 ng/mL while a 10 mg/kg p.o. dose of GX led to plasma levels of 37 ng/mL. The authors indicated that the observed superior in vivo potency for UCI-50027 (vs. GX) was due to an increased oral bioavailability, compensating for the lower in vitro potency of UCI-50027. Karout48 and coworkers tested a set of synthetic derivatives of allopregnanolone to potentially identify NASs with increased efficacy, in particular their neuroprotective activity against the toxic effects of amyloid beta 42 peptide (Ab42), a major component of amyloid plaques. The authors focused their research on 3-O-allyl analogs (Fig. 6) to increase metabolic stability vs allopregnanolone (1). Compound 1 or its analogs were added to neurosphere cultures 1 h before Ab42 was applied for 2 days. Fluorometric measurement of caspase-3/7 activity was used to determine the extent of apoptotic cell death. Under these conditions, the Ab42-induced increase in fluorescence was significantly reduced (40%) in the presence of 1. The 3-O-allyl analogs, irrespective of orientation of the substituent, efficiently protected against Ab42 toxicity, reducing apoptotic activity to basal levels. The authors postulated that since both isomers showed equal neuroprotective effects, the mechanism of action could be off target rather than mediated through the GABAAR. Sage Therapeutics has also reported on a series of nor-19 pregnanolone bearing a heterocycle substituent at C21. These efforts resulted in the identification of SGE-516,49 a PAM of both gamma and delta- containing GABAAR (Fig. 7, Table 2). The broad GABAAR activity differentiates NASs like SGE-516 from benzodiazepines, a class of anticonvulsants which have been shown in vitro to selectively target gamma-subunit containing GABAARs. In addition, SGE-516 has PK properties suitable for chronic oral dosing (Table 2). Compound SGE-516 has been profiled in numerous in vivo preclinical models of seizure activity. In mice, SGE-516 protected against acute seizures in the PTZ-induced chemo-convulsant seizure model (ED50 = 2.0 mg/kg, ip) and the 6-Hz psychomotor seizure model (ED50 = 3.8 mg/kg). In addition, SGE516 demonstrated anticonvulsant activity in the mouse corneal electrical kindling assay, a model thought to be relevant for chronic epilepsy. After oral administration, SGE-516 increased the latency to clonic and tonic seizures in a dose-dependent fashion (ED50 = 2.82 mg/kg) and prevented death after PTZ administration (Table 3).50 For the last several years, there has been an increasing interest in extrasynaptic GABAAR activity and its translation into potentially differentiated pharmacology through tonic modulation not previously studied for neuronal excitability.51–54 The importance of this tonic regulation is reflected in the potential therapeutic Fig. 8. Reddy’s description of functional activation of extrasynaptic d GABAARs.57 M.-J. Blanco et al. / Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 67 Fig. 12. Formula used to estimate IC50 by Kudova et al. Fig. 9. Nor-19-pregnanolone analog SGE-872. Table 5 In vitro ephys GABAA pharmacology of SGE-872.49. a1b2c2 EC50 (nM)/Emax (%) a4b3d EC50 (nM)/Emax (%) >3000/>744 178/858 Fig. 10. Representatives of the two classes of NAS negative modulators of NMDARs. opportunities of extrasynaptic GABAARs as anesthetics, sleep-promoting drugs, and alcohol addiction disorders. NASs have shown greater sensitivity to extrasynaptic GABAARs55 in comparison to other compounds such as benzodiazepines, leading to their importance in stress-, ovarian cycle-, and pregnancy-related mood disorders. Additionally, disruptions in network dynamics associated with schizophrenia, epilepsy, and Parkinson’s disease might involve alterations in the tonic GABAA receptors-mediated conductance.56 These potential therapeutic opportunities have increased the relevance to identify suitable NAS tools (see Table 4). In 2016, Reddy and colleagues reported some key structural features for the identification of a NAS pharmacophore to modulate extrasynaptic GABAAR–mediated tonic inhibition in murine dentate gyrus granule cells (DGGCs) in the hippocampus using well known natural and synthetic NASs (Fig. 8).57 The pharmacophore map summarizes the correlation between NAS modulation of tonic current and anticonvulsant profiles in the 6-Hz seizure model. The authors built this correlation after performing experiments in granule cells from d-knockout mice showing that NAS potentiation of tonic currents was completely (95%) diminished. Furthermore, the authors confirmed the NAS sensitivity of dGABAARs at the systems level using anticonvulsant 6-Hz seizure mice model. As a result, the authors highlighted that the 3a-OH (24) substitution was crucial for extrasynaptic receptor functional activity, since the 3b-OH epimer (25) was inactive in activating tonic currents. Allopregnanolone (1) exhibited the highest potency and maximal efficacy in promoting tonic currents. Modifications at the C20 position of NAS, (tetrahydrodeoxycorticosterone, THDOC, 26) Fig. 13. Various pregnane derivatives investigated for inhibitory activity. significantly changed the transduction kinetics of tonic current activation. It is important to emphasize the moderate correlation between an ex-vivo slice assay in DGGCs and an in vivo assay (6-Hz seizure model) with compounds that might have different PK profiles. The NAS-mediated increase in phosphorylation and surface levels of extrasynaptic GABAARs seen with THDOC (26) leads to an increase in tonic current. This is an additional mechanism by which NASs can increase the inhibitory tone in the brain in addition to the known allosteric effect. Modgil and collaborators58 evaluated various NASs (1, 6 and SGE-516) on their ability to increase tonic current by promoting GABAAR phosphorylation and membrane trafficking dependent on protein kinase C (PKC) activity. A sustained increase in tonic current was observed following exposure to 1, or SGE-516 however no increase in tonic current was observed with exposure to 6. In agreement with the observations of an increased tonic current, 1 and SGE-516 increased the phosphorylation and surface expression of the b3 subunit-containing GABAARs. The authors postulate that those results open an interesting area of potential therapeutic targets aimed at modulating the trafficking of a particularly important subset of GABAARs. In 2015, Sage Therapeutics reported the identification of SGE872,49 a 5-N azabenzopyrazole NAS with a strong preference for a4b3d GABAA vs. a1b2c receptors (>17-fold, Fig. 9, Table 5) as a potential tool molecule to investigate extrasynaptic pharmacology. Preclinical NMDAR NASs. In addition of GABAAR modulators, there have been recent advancements within the context of NMDARs. While there has been significant activity outside of steroids,59 the last section of this review will focus specifically on the NAS genre of modulators. The NMDARs are non-selective cation channels, that play an important role in excitatory synaptic transmission and several forms of synaptic plasticity.60 NMDARs belong to the ionotropic glutamate receptor family but unlike most receptors, NMDARs contain a magnesium ion which blocks the channel and requires dissociation prior to activation. The ion is removed through activation of co-localized AMPA receptors which allows the influx of sodium ions thereby increasing internal positive charge resulting in electrostatic repulsion liberating the magnesium ion. Upon binding of glutamate and glycine or D-serine to Fig. 11. Key compounds demonstrating the effect of C17 substitution. 68 M.-J. Blanco et al. / Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 Fig. 14. Amide-based steroidal inhibitors. Fig. 15. SAR of NAS-like perhydrophenanthrene analogs. the ligand binding domain (LBD) of NMDAR, tertiary changes results in the passage of calcium ions into the postsynaptic neuron. The influx of calcium leads to downstream cellular cascades which strengthens signaling through increasing expression of signaling receptors and triggering development of more synapses in the same pathway. The signaling pathway, therefore, is strengthened only when sufficiently stimulated; this is the basic principle behind long-term potentiation (LTP) and a hypothesized biological basis of learning and memory. Given NMDARs central role in synaptic physiology, excessive activation has been implicated in excitotoxicity, central to the damage of neurological disorders such as stroke, traumatic brain injury (TBI), neurodegeneration and depression. In addition, NMDAR hypofunction has been associated with schizophrenia, Alzheimer’s diseases and dementia.61 The NMDAR is a heterotetramer comprised of a dimer-ofdimers arising from two N1 and two N2(A–D) or N3(A, B) subunits. There are four domains, the amino terminal domain (ATD), the ligand binding domain (LBD), the transmembrane domain (TMD) and the cytoplasmic carboxy terminal domain (CTD). The TMD contains a series of four a-helices, M1-4, which undergo structural changes in response to conformational movement upon agonist binding to the LBD therefore coupling ligand association and channel gating.62 NASs that modulate NMDARs can be categorized in two general classes, the oxysterols, which arise from cholestane hydroxylation4 and pregnane derivatives that bear a charge group at C3 such as a sulfate, hemisuccinate, glutamate, etc. Representative examples, 24 (S)-hydroxycholesterol (2, 24HC) and pregnenolone sulfate (28, PS, pregn-5-en-3b-ol-20-one 3b-sulfate), are shown in Fig. 10. Modulators from both classes can act as either PAMs or NAMs. While the exact binding sites of these NASs are not fully understood, chimera experiments that swapped domains between rat NMDAR and GluK2 kainate receptors revealed that 2 requires the TMD and 28 requires the TMD and LBD of the NMDAR for potentiation.63 The authors suggest that while more extensive mechanistic studies are required to elucidate the exact site of binding for the NAS modulators, these regions could contain the site of binding. Given the strong association between NMDAR hyperactivity and neurological disorders, NASs inspired by pregnanolone sulfate (PAS, 29, Fig. 11), an endogenous inhibitor of NMDAR, have been investigated.64 The amphiphilic nature of this class of NASs gives rise to complications stemming from the equilibrium between single vs aggregate forms in solution. The authors, therefore assert that the formula below, which assumes 100% inhibition at saturating inhibitor concentrations, accurately estimates IC50. The formula employed is shown in Fig. 12, where [compound] is the steroid concentration, II is the relative degree of inhibition and h (the apparent Hill coefficient) is fixed at 1.2. From the SAR, dramatic changes in potency were observed with modification at the C17 position. Improvements in potency were observed up to 273-fold (31) over 29 (Fig. 11) and generally tolerant to halogenation and unsaturation. As a tool molecule, compound 30 (0.60 mM) demonstrated activity against all isoforms of GluN2(A–D). In addition, 30 is active when evaluating NMDA, AMPA and GABA receptor response in hippocampal neurons. The authors also presented a strong correlation between experimental DGexp values and calculated logP and logD. Given this relationship, the authors concluded that the potency within this class could be dictated by protein affinity as well as changes in membrane concentration proximal to the receptor. Nevertheless, the same group suggested that the site of action of 29 is the extracellular vestibule of the ion channel pore65 and is consistent with the chimera work described above.63 In addition to sulfate NAS inhibitors, charged ester derivatives such as those in Fig. 13, have also been shown to selectively inhibit tonic versus phasic NMDAR signaling.66 The authors indicated that within the context of neurological disease treatment, a tonic inhibitor may offer a greater therapeutic index due to the association between synaptic/phasic inhibition and psychomimetic symptoms. This investigation revealed that as the chain length increases, so does potency and tonic selectivity. The steady-state potencies ranged from 10–60 lM on GluN1/GluN2B receptors where the general trend was greater potency with increased chain length [PAS (29) was measured at 23 lM]. Ultimately, the hemipimelate analog (PA-hPim, 37) was determined to have a potency of 7.4–12 mM for tonic inhibition of native NMDARs. In contrast, this compound did not inhibit synaptically activated NMDARs. Compound 37 was further tested in several in vivo models evaluating psychomimetic symptoms such as the locomotor activity, passive avoidance task and the Y-maze spontaneous alteration task assays. Hyperlocomotion was not observed with 1 or 10 mg/kg of 37. In addition, as opposed to control MK-801, it had no effect in the step-through passive avoidance test or on spontaneous alteration in the Y-maze. When advanced to a intrahippocampal NMDA infusion study, 37 reversed learning deficits. In totality, the authors suggested that this body of work emphasized the potential of NASs as novel therapeutics. Influenced by this study as well as those with 29, analogs of pregnanolone glutamate (PAG, 38, Fig. 14)67 were synthesized, attempting to improve the pharmacokinetic profiles.68 With the four compounds illustrated in Fig. 14, improvements in potency M.-J. Blanco et al. / Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 Fig. 16. Structure of tetrol. Fig. 17. Structures of NMDAR PAMs SGE-201 and SGE-301. were seen, in particular with compounds 41 and 42. The permeability, as measured in the Caco-2 assay, was as low as 0.22 ± 0.060 106 cm/s and did not exceed 5.85 ± 0.060 106 cm/s with recoveries that were typically <50%. Compounds 39–42 were further assessed in a spontaneous locomotor activity test and all compounds had significant effects on distance travelled except for compound 41. The authors suggested that the reduced effect in relation to the other analogs was due to lower CNS penetration stemming from poor Caco-2 permeability. Compound brain concentrations were not provided to support these assumptions. Advances in sulfate and glutamate-NAS research has prompted further investigation of this scaffold through modifications of the tetracyclic core. Deconstruction of the D-ring was examined by the synthesis of perhydrophenanthrenes (compounds 43–46) as illustrated in Fig. 15 which highlights the most potent compounds reported.69 The potency values observed, which were measured in HEK293 cells transfected with rat GluN1 and GluN2B subunits, were similar to pregnanolone sulfate (29, 24.6 mM, Fig. 11). In general, methyl ether analogs (43–44, R2 = CH2OCH3 in Fig. 15) were not as potent as the unsubstituted analogs (45–46, R2 = H). Further in vitro evaluation of 45 revealed that it was not subtype selective (GluN2A-D) and less potent against AMPA receptor responses (83 mM) in hippocampal neurons. Conversely, 45 was more potent and effective in inhibiting GABAAR responses (3.1 lM). The authors concluded, therefore, that the neuroid D-ring is critical for NMDAR/GABAR selectivity and provides potential avenues for optimization. While marine steroid 24-methylenecholestane-3b,5a,6b,19tetrol70 (tetrol, 47, Fig. 16) has previously been characterized as an anti-cancer agent, more recently, it has been determined to be an NMDA negative modulator.71 Tetrol inhibits NMDA-induced calcium concentration (IC50 = 7.8 mM) and NMDA current in cortical neurons (IC50 = 10.3 mM). Tetrol was synthesized in 10 steps from hyodeoxycholic acid (HDCA) which enabled further evaluation in models of neuronal injury. For instance, 10 mM of tetrol increased the number of surviving cerebellar granule neurons when exposed to 200 mM glutamate, a concentration that kills neurons in the control. Staining experiments employing fluorescin diacetate revealed that improvements in viability were observed at concentration as low as 2.5 mM. The authors also demonstrated that 47 significantly reduce the infarction volume by roughly 30% in a rat permanent focal cerebral ischemia model induced by middle cerebral artery occlusion (MCAO). In addition to the negative modulators described above, considerable progress has been made towards identifying PAM NASs at 69 the NMDAR. Through a screen of endogenous oxysterols and cholesterol metabolites, Paul et al.4 reported the identification of 2 (Fig. 1) as a PAM with an estimated EC50 of 1.2 mM in hippocampal neurons. In addition, two synthetic analogs, SGE-201 and SGE301 (48–49, Fig. 17), were shown to have PAM activity and the authors suggested that while the binding site of 2 is disparate from PS (28, Fig. 10), it is likely shared with these structural homologous analogs. Compounds 2 and 48 at 1 mM each potentiated NMDAR EPSCs which were pharmacologically isolated by NBQX and gabazine, agents that antagonize the AMPA and GABA receptors respectively, thereby eliminating confounding signaling. Compounds 2 and 48 enhanced long-term potentiation (LTP) in hippocampal slices and reversed synaptic plasticity deficits induced by ketamine and also reverses MK-801 induced deficits in spontaneous alterations in a mouse Y-maze test. Additionally, 49 rescues social interaction deficits and novel object recognition in PCP treated rats. As an extension of this work, Sage Therapeutics has recently advanced the first NMDAR PAM, SAGE-718, into phase 1 clinical trials.23 In summary, there has been tremendous interest in the development of novel NASs during the past 5 years. There has been a continuous flow of new NASs entering clinical studies with some showing promising positive results in phase 2 and 3. Although we have emphasized the importance of GABAA and NMDA receptor modulators, opportunities exist to explore the effects of NASs on alternative intracellular targets and other signaling pathways intersecting with actions at the membrane with GABA, glutamate or other ion channels. NASs have demonstrated specific interactions with multiple CNS targets and represent an attractive starting point for novel therapeutics. Acknowledgements We thank Dr. A. Robichaud and Dr. J. Doherty for valuable discussions during the elaboration of this review. References 1. Baulieu EE. Steroid hormone regulation of the brain. In: Fuxe K, Gustafsson J-Å, Wetterberg L, eds. Steroid hormones in the brain: several mechanisms, 34. Stockholm: Pergamon Press Ltd; 1981:3–4. 2. Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosci Biobehav Rev. 2013;37:109. 3. Reddy DS, Estes WA. Trends Pharmacol Sci. 2016;37:543. 4. Paul SM, Doherty JJ, Robichaud AJ, et al. J Neurosci. 2013;33:17290. 5. Serrano, E., Kanner, A. F1000Prime Rep. 2015, 7. 6. Panico R, Powell WH, Richer JC. IUPAC commission on the nomenclature of organic chemistry: a guide to IUPAC nomenclature of organic compounds, vol. 8. Oxford UK: Blackwell Scientific Publications Ltd.; 1994. 71. 7. Clarke RS, Dundee JW, Carson IW. Proc R Soc Med. 1973;66:49. 8. Monagle J, Siu L, Worrell J, Goodchild CS, Serrao JM. Anesth Analg. 2015;121:914. 9. McNeill HG, Clarke RS, Dundee JW, Briggs LP. Anaesthesia. 1981;36:592. 10. Drug Profile: ORG 20599. <http://adisinsight.springer.com/drugs/800003651>; 2001 Accessed 16.08.17. 11. Caperelli, L. <http://ir.marinuspharma.com/releasedetail.cfm?releaseid= 975400>; Published 2016 Accessed 16.08.17. 12. Bixo M, Ekberg K, Poromaa IS, et al. Psychoneuroendocrinology. 2017;80:46. 13. Elia AE, Lalli S, Monsurro MR, et al. Eur J Neurol. 2016;23:45. 14. Ling J, Yu Y, Zhu J, et al. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1031:214. 15. Comparison of Yuxintine with Placebo in Treatment of MDD. <https://clinicaltrials.gov/ct2/show/NCT02395263?term=Yuxintine&cntry1=ES %3ACN&rank=1>; 2015 Accessed 16.08.17. 16. A Study to Evaluate SAGE-547 in Patients with Moderate Postpartum Depression. <https://clinicaltrials.gov/ct2/show/NCT02942017>; 2016 Accessed 16.08.17. 17. A Study to Evaluate SAGE-547 in Patients with Severe Postpartum Depression. <https://clinicaltrials.gov/ct2/show/NCT02614547>; 2015 Accessed 16.08.17. 18. A Study with SAGE-547 for Super-Refractory Status Epilepticus. <https://clinicaltrials.gov/ct2/show/NCT02477618>; 2015 Accessed 16.08.17. 19. A Study to Evaluate SAGE-217 in Subjects with Moderate to Severe Major Depressive Disorder. <https://clinicaltrials.gov/ct2/show/NCT03000530>; 2016 Accessed 16.08.17. 70 M.-J. Blanco et al. / Bioorganic & Medicinal Chemistry Letters 28 (2018) 61–70 20. A Study to Evaluate SAGE-217 in Subjects with Parkinson’s Disease. <https://clinicaltrials.gov/ct2/show/NCT03000569>; 2016 Accessed 16.08.17. 21. A Study to Evaluate SAGE-217 in Subjects with Essential Tremor. <https://clinicaltrials.gov/ct2/show/NCT02978781>; 2016 Accessed 16.08.17. 22. A Study to Evaluate SAGE-217 in Subjects with Severe Postpartum Depression. <https://clinicaltrials.gov/ct2/show/NCT02978326>; 2016 Accessed 16.08.17. 23. Cox, P. <http://investor.sagerx.com/news-releases/news-release-details/sagetherapeutics-announces-initiation-phase-1-development-and>; 2017 Accessed 16.08.17. 24. Thurman DJ, Beghi E, Begley CE, et al. Epilepsia. 2011;52:2. 25. Lanza di Scalea T, Pearlstein T. Psychiatr Clin North Am. 2017;40:201. 26. Vang S, Longley K, Steer CJ, Low WC. Glob Adv Heal Med. 2014;3:58. 27. Fava M, Kendler KS. Neuron. 2000;28:335. 28. Xu C, Teng J, Chen W, et al. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:1402. 29. Kanes SJ, Colquhoun H, Doherty J, et al. Hum Psychopharmacol Clin Exp. 2017;32:e2576. 30. Vaitkevicius H, Husain AM, Rosenthal ES, et al. Ann Clin Transl Neurol. 2017;4:411. 31. Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Epilepsy Res. 2015;111:85. 32. Cox, P. <http://www.businesswire.com/news/home/20170912005509/en/ Sage-Therapeutics-Reports-Top-Line-Results-Phase-3>; 2017 Accessed 21.11.17. 33. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Lancet. 2017;390:480. 34. Ellenbogen A, Raines S, Kanes S. Neurology. 2016;86:P4–297. 35. Maguire J, Mody I. Neuron. 2008;59:207. 36. Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Obstet Gynecol. 2005;106:1071. 37. Cox, L. <http://investor.sagerx.com/news-releases/news-release-details/sagetherapeutics-announces-brexanolone-achieves-primary>; 2017 Accessed 21.11.17. 38. Martinez Botella G, Salituro FG, Harrison BL, et al. J Med Chem. 2017;60:7810. 39. Robichaud, A. J. Abstr Pap 252nd ACS Natl Meet Expo, Philadelphia. 2016. 40. Irwin RW, Solinsky CM, Brinton RD. Front Cell Neurosci. 2014;8. 41. Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. Trends Neurosci. 2010;33:299. 42. Durham TB, Blanco M-J. Bioorg Med Chem Lett. 2015;25:998. 43. Carver CM, Reddy DS. Psychopharmacology. 2013;230:151. 44. Martinez Botella G, Ackley MA, Salituro FG, Doherty JJ. Annu Rep Med Chem. 2014;49:27. 45. Rey M, Kruse MS, Alvarez LD, et al. Exp Neurol. 2013;249:49. 46. Kasal A, Buděšínský M, Mareš P, et al. Steroids. 2016;105:12. 47. Hogenkamp DJ, Tran MB, Yoshimura RF, Johnstone TB, Kanner R, Gee KW. Psychopharmacology. 2014;231:3517. 48. Karout M, Miesch M, Geoffroy P, et al. J Neurochem. 2016;139:782. 49. Martinez Botella G, Salituro FG, Harrison BL, et al. J Med Chem. 2015;58:3500. 50. Hammond RS, Althaus AL, Ackley MA, et al. J Epilepsy Res. 2017;134:16. 51. Carlson SL, Bohnsack PJ, Patel V, Morrow LA. J Pharmacol Exp Ther. 2016;356:148. 52. Clossen BL, Reddy DS. J Neurosci Res. 2017;95:1906. 53. Errington AC. Extrasynaptic GABAA receptors. In: Errington AC, Di Giovanni G, Crunelli V, eds. New York: Springer; 2014:1–14. 54. Fritschy J, Panzanelli P. Extrasynaptic GABAA Receptors. In: Errington A, Di Giovanni G, Crunelli V, eds. New York: Springer; 2014:15–32. 55. Abramian AM, Comenencia-Ortiz E, Modgil A, et al. Proc Natl Acad Sci USA. 2014;111:7132. 56. Kim YS, Yoon B. Exp Neurobiol. 2017;26:122. 57. Carver C, Reddy DS. J Pharmacol Exp Ther. 2016;357:188. 58. Modgil A, Parakala ML, Ackley MA, Doherty JJ, Moss SJ, Davies PA. Neuropharmacology. 2017;113:314. 59. Strong KL, Jing Y, Prosser AR, Traynelis SF, Liotta DC. Expert Opin Ther Pat. 2014;24:1349. 60. Paoletti P, Bellone C, Zhou Q. Nat Rev Neurosci. 2013;14:383. 61. Zorumski CF, Izumi Y. Neurosci Biobehav Rev. 2012;36:989. 62. Traynelis SF, Wollmuth LP, Mcbain CJ, et al. Pharmacol Rev. 2014;62:405. 63. Wilding TJ, Lopez MN, Huettner XE. J Neurosci. 2016;36:8815. 64. Kudova E, Chodounska H, Slavikova B, et al. J Med Chem. 2015;58:5950. 65. Vyklicky V, Krausova B, Cerny J, et al. Sci Rep. 2015;5:10935. 66. Vyklicky V, Smejkalova T, Krausova B, et al. J Neurosci. 2016;36:2161. 67. Holubova K, Nekovarova T, Pistovcakova J, Sulcova A, Stuchlík A, Vales K. Front Behav Neurosci. 2014;8:130. 68. Adla SK, Hubalkova P, Krausova B, et al. Steroids. 2017;117:52. 69. Slavikova B, Chodounska H, Nekardova M, et al. J Med Chem. 2016;59:4724. 70. Duh C-Y, Wang S-K, Chu M-J, Sheu J-H. J Nat Prod. 1998;61:1022. 71. Leng T, Liu A, Wang Y, Chen X, Zhou S, Li Q. Steroids. 2016;105:96.