CENTROSOME AMPLIFICATION AND POSSIBLE ROLE IN MAMMARY CANCER a
advertisement

CENTROSOME AMPLIFICATION AND
POSSIBLE ROLE IN MAMMARY CANCER
Ana Maria Abreu Velez MD, phD, DrSc
BREAST CANCER
-All women are at risk for getting breast cancer,
especially. Aging increases the risk.
-Brest cancer risk over lifetime about 14%.
-1/7 women will get breast cancer over a 90-year life
span.
- Male breast cancer is an uncommon disease.
CENTROSOME AMPLIFICATION AND
POSSIBLE ROLE IN MAMMARY CANCER
Breast profile:
A ducts
B lobules
C dilated section of duct to hold milk
D nipple
E fat
F pectoralis major muscle
G chest wall/rib cage
Enlargement:
A normal duct cells
B basement membrane
C lumen (center of duct)
Brest cancer
Sub-classification:
DH: Ductal hyperplasia.
ADH: atypical ductal hyperplasia.
DCIS: Invasive ductal carcinoma.
ALH: atypical lobular hyperplasia.
ILC: Invasive lobular carcinoma.
Normal breast with lobular carcinoma in situ
(LCIS)
A Ducts
B Lobules
C Dilated section of duct to hold milk
D Nipple
E Fat
F Pectoralis major muscle
G Chest wall/rib cage
A Normal lobular cells
B Lobular cancer cells
C Basement membrane
Stage 0: This stage is used to describe non-invasive breast
cancer e.g: {Lobular Carcinoma In Situ (LCIS) and Ductal
Carcinoma In Situ (DCIS).
LCIS is generally considered to be a pre-cancerous condition.
It is lobular because the cancer is confined to the lobules (glands
that make milk).
DCIS, is not an invasive cancer. It stays inside the milk duct of
the breast in which it started. It can grow to cover a small or
large area of the breast. But it does not spread OUTSIDE the
duct.
Invasive Brest Cancer
Stage IIIA: invasive breast cancer in which a tumor of any size
has spread to the breast skin, chest wall, or internal mammary
lymph nodes (located beneath the breast right under the ribs).
Stage IIIB includes INFLAMMATORY BREST CANCER, a
very uncommon but very serious, aggressive type of breast
cancer. The most distinguishing feature of inflammatory breast
cancer is redness involving part or all of the breast. ("peau
d'orange").
Stage IV: This stage includes invasive breast cancer in which a
tumor has spread beyond the breast, underarm, and internal
mammary lymph nodes, and collarbone), lungs, liver, bone, or
brain.
COMMON TERMINOLOGY
Blastocyst: a stage in early embryonic developments in which the cells form
a sphere with a fluid-filled cavity in the centre.
Breeding Stock: a breeding colony of animals whose phenotype has been
well described.
Chimera: an animal produced experimentally by combining cells of different
genetic origins. In mouse genetics, targeted mutations produced in embryonic
stem cells are recovered by breeding chimeric mice resulting from the mixture
of embryonic stem cells with a genetically distinct blastocyst.
Clone: a genetic copy of another living or dead animal. It is not a twin derived
by the fertilization of an egg by a sperm (see Somatic cell nuclear transfer and
Cloning).
COMMON TERMINOLOGY
Cloning: refers to the propagation of genetically exact duplicates
of an organism by means other than sexual reproduction.
Electroporation: introduction of DNA into cells by means of
electrical pulses.
Epigenetic: changes in gene expression that occur without
changing the DNA sequence of genes.
Exogenous: refers to a gene taken from an organism and
introduced into the DNA of a target animal.
Gene expression: the process by which the genetic information
or blueprint in genes is transformed into the structure and
function of an organism.
COMMON TERMINOLOGY
Genotype: the genetic makeup, as distinguished from the
physical appearance, of an organism or a group of organisms.
Genetic modification (of animals): the use of any technique
for the modification of genes or other genetic material but not
including the use of natural processes such as sexual
reproduction.
Heterozygous: describes the situation where cells or organisms
carry two different versions of a given gene, one from each
parent, at the corresponding site on chromosomes.
Homozygous: describes the situation where cells or organisms
carry the same versions of a given gene, one from each parent,
at the corresponding site on chromosomes.
COMMON TERMINOLOGY
Hybrid: an organism that is the offspring of genetically
dissimilar parents or stock, especially offspring produced by
breeding animals of different breeds or species.
Imprinting: refers to chemical marks on the DNA from the
dam and sire so that only one copy of a gene (either the
maternal or paternal gene) is activated. The chemical mark on
the DNA is usually methylation and imprinting is a form of
epigenetic inheritance.
Knock-in: the introduction by gene targeting of DNA
sequences at a specific site.
Knock-out: a mutation in which the target gene is inactivated.
COMMON TERMINOLOGY
Microinjection: also called pronuclear injection, is when DNA is
injected into the nucleus of a single cell embryo using a very
fine needle.
Mutation: a permanent transmissible change in the genetic
code. It can be an insertion or deletion of genetic information, or
an alteration in the original genetic information. Mutations can be
caused by many factors including environmental insults such as
radiation and mutagenic chemicals.
Oocyte: a female germ cell that is in the process of growing into
an egg.
COMMON TERMINOLOGY
Phenotype: the observable physical, behavioral, physiological or
biochemical characteristics of an organism, as determined by
both genetic makeup and environmental influences.
3Rs: Reduction in the number of animals used, Replacement
of animals with other methods and Refinement of techniques
used to reduce the impact on animals when animals are used for
scientific purposes.
Re-program, reprogramming: refers to processes where the
genetic material in body cells, which is geared to express the
particular characteristics of the differentiated tissue from which it
comes (e.g. muscle or nervous tissue), is returned to a state
where it can once again differentiate into various tissue types.
COMMON TERMINOLOGY
Somatic cell: any cell of an animal other than a reproductive
cell.
Somatic cell nuclear transfer: the technique of inserting a
nucleus of a somatic cell from one of the body's tissues, other
than a germ cell, into an egg that has had its nucleus removed.
Targeted mutagenesis: (Knock-out).
Transgene: the gene(s) transferred into another organism.
Transgenic: refers to an organism containing a transgene.
Vector: a vehicle such as a modified plasmid, virus or DNA
molecule, capable of being replicated and bearing cloning sites
for the introduction of foreign DNA, which is used to
introduce foreign DNA into host cells.
COMMON TERMINOLOGY
The term transgenesis refers to insertion of exogenous DNA into
cells, typically fertilized eggs.
DNA might be inserted into cells using microinjection,
electroporation or certain non-pathogenic viruses.
Blastocysts containing manipulated cells may or may not be
cultured in vitro before they are implanted into the oviducts or
uterus of surrogate mothers.
The inserted DNA successfully incorporates into the
chromosomes of only a small percentage of embryos. The
DNA incorporates at different genetic locations and a different
number of copies of the DNA may incorporate in different
embryos. Not all modified fertilized eggs develop into live born
transgenic animals.
Basic questions
– What transgenic mice and /or knockout are we using?
– How were they created?
– Are the mutagenesis stable and or the transgenic?
– What type of histopathologic pattern is producing each
gene by itself after stimulation?
– -What types of changes are expected in the animal
genoma and in the centrosome per se?
- Do the antibodies that we are using detect in similar
fashion the intact cells, versus cells that have go to
apoptosis?
ANIMAL MODELS IN OUR LAB
E2F knockouts mice SERIES:
E2F1-/- 1
E2F2-/- 2
E2F3-/- 3
E2F4-/- 4
E2F5-/- 5
E2F3flox 3F
P53 P
P53flox PF
Recent findings demonstrate that Ca2+-activated centrin forms a
complex with the visual G-protein transducin in photoreceptor
cells. This Ca2+-dependent assembly of G-proteins with centrin
is a novel aspect of the supply of signaling proteins in sensory
cells, and a potential link between molecular translocations and
signal transduction in general.
This superfamily which can be divided into two subfamilies,
probably associated with different functions: one related to
Chlamydomonas reinhardtii centrin, CrCenp, and the other,
represented by Saccharomyces cerevisiae isoform, ScCdc31p.
ESTs encoding the two isoforms (BeCen1 and BeCen3) from the
chytridiomycete Blastocladiella emersonii were isolated, and
expression of the CrCenp-type centrin, BeCen1, was analyzed
throughout the fungus life cycle. Becen1 mRNA levels increase
transiently during sporulation and protein levels present a similar
pattern. Immunolocalization studies seem to localize BeCen1 at
the basal body zone and in the cytoplasm surrounding the
nuclear cap, a zoospore organelle.
Centrin is a calcium-binding phosphoprotein, and flagellar basal
apparatus.
IIF in human and rat retinas reveal centrin localization in two
distinct cellular structures: at centrosomes of nonciliated neuronal
cells as well as in basal bodies, and the connecting cilium--of
photoreceptor cells. Western blot analyses of mammalian retinal
proteins show two closely migrating centrin (20 kDa).
Using isoform specific primers in PCR, the expression of two related but
distinct forms of centrin (centrin 1 and centrin 2), can be identified in the
retina of human and rat as well as in the testis where cilia are present.
However, only one isoform (centrin 2) is expressed in nondifferentiated,
nonciliated retinal cells (retinoblastoma cells), as well as in rat liver, skeletal
muscle, and cardiac muscle. These observations suggest centrin 2 message
may be universally expressed while centrin 1 message may be restricted to
retina and testis which contain cells that have differentiated cilia or flagella.
“Centrin 1 and/or centrin 2 are involved in ciliary beating”
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE
IN MAMMARY CANCER
Interaction assays revealed binding potential of the four
centrin isoforms to Gtbetagamma heterodimers. High
affinity binding to Gtbetagamma and subcellular
localization of the centrin isoforms Cen1 and Cen2 in the
connecting cilium indicated that these isoforms contribute
to the centrin-transducin complex and potentially
participate in the regulation of transducin translocation
through the photoreceptor cilium. Binding of Cen2 and
Cen4 to Gbetagamma of non-visual G-proteins may
additionally regulate G-proteins involved in centrosome
and basal body functions.
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE
IN MAMMARY CANCER
Known facts:
Enforced expression of two viral proteins, SV40 T-Ag and t-Ag, and
two mammalian proteins, RasG12V and hTERT .
-Ag binds to and inactivates the tumor suppressors Rb and p53,
two genes commonly disrupted in human cancers, which allows
cells to evade antiproliferative and apoptotic signals. Rb function
can also be disrupted in human cells vis-a-vis constitutive
phosphorylation by an activated cyclin/cyclin-dependent kinase
(CDK) complex and both overexpression of cyclin D and activation
mutants of CDK4 have been found in human cancers p53 function
can be impeded in human cells by expression of p53DD, a
dominant-negative form of the protein.
THE PROBLEM USING MICE MODELS FOR
UNDERSTANDING THE HUMAN COUNTERPART:
Great progress has been made in identifying individual changes that
foster these various phenotypes, such as loss of tumor suppressors,
activation of oncoproteins, restoration of telomerase activity, etc.
However, it has been more challenging to reassemble how these
pathways collectively drive the tumorigenic process. While mice models
have been valuable in this regard, genetic alterations characteristic of
specific human cancers often do not yield the same type of cancer
in mice, mice are prone to different types of cancers, and their cells
are far more easily transformed compared with humans.
The real needs: to recreate a phenotype(s) that include the ability to
proliferate independent of external cues while also overcoming
antiproliferative and apoptotic signals, in addition to cellular immortality,
angiogenesis, and ultimately invasion and metastasis.
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE
IN MAMMARY CANCER
The rapid introduction of these transgenes and
formation of tumors supports the argument that
expression of hTERT, p53DD, cyclin D1,
CDK4R24C, c-MycT58A, and H-RasG12V is
sufficient to drive normal human cells to a
tumorigenic state.
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE
IN MAMMARY CANCER
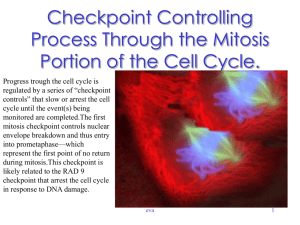
Centrosome and its critical role in cell division or mitosis.
The centrosome is an integral component of the eukaryotic cell
cycle machinery, yet very few centrosomal proteins have
been fully characterized to date.
Centrins are highly conserved calcium-binding proteins
involved in the nucleotide-excision repair pathway as a subunit
of the heterotrimer including the Xeroderma pigmentosum
(XPC) and Rad23 proteins bind ubiquitinated substrates and the
proteasome (hHR23B) proteins. consistent with an important
role in protein degradation. Although human Rad23 proteins
(hHR23A and hHR23B) have redundant roles in DNA repair.
and nuclear mRNA export.
Centrins are calmodulin-like proteins.
CENTROSOMES
Centrosomes: are dynamic organelles involved in many
aspects of cell function and growth.
Act as microtubule organizing centers.
Provide a site for concerted regulation of cell cycle progression.
Centrin, is conserved in Eukaryotes.
Experimental analysis has provided an outline to describe
centrosome duplication, and numerous centrosome components
have been identified. Even so, more work is needed to provide
a detailed understanding of the interactions between
centrosome components and their roles in centrosome function
and duplication.
Precise duplication of centrosomes once during each cell cycle
ensures proper mitotic spindle formation and chromosome
segregation. Defects in centrosome duplication or function are
linked to human diseases including cancer.
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE IN
MAMMARY CANCER
Basic concepts: Centrosomes, the major microtubule-organizing
centres (MTOCs) of animal cells, are comprised of a pair of centrioles
surrounded by pericentriolar material (PCM). Early in the cell cycle,
there is a single centrosome, which duplicates during S-phase to direct
bipolar spindle assembly during mitosis.
Centrosome: The centrosome, play a major role on microtubule
organization center. It plays a vital role in mitotic fidelity, ensuring
establishment of bipolar spindles and balanced chromosome
segregation.
Centrosome duplication: this phenomenon occurs only once during
the cell cycle and is therefore highly regulated. During the progression
of cell cycle, the pair of centrosomes help to segregate sister
chromatides, resulting in equal segregation of chromosomes.
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE IN MAMMARY
CANCER
During mitosis, two centrosomes form spindle poles and direct the
formation of bipolar mitotic spindles, which is an essential event for
accurate chromosome segregation into daughter cells.
Centrosome amplification (CA):
The presence of more than two centrosomes (centrosome
amplification), severely disturbs mitotic process and cytokinesis via
formation of more than two spindle poles, resulting in an increased
frequency of chromosome segregation errors (chromosome instability).
Destabilization of chromosomes by CA aids acquisition of further
malignant phenotypes, hence promoting tumor progression.
Centrosome amplification occurs frequently in almost all types of
cancer, and is considered as the major contributing factor for
chromosome instability in cancer cells.
Centrosomes components
CP110 interacts with two different Ca2+-binding
proteins, calmodulin (CaM) and centrin.
Binding experiments reveal a direct, robust interaction
between CP110 and CaM. Native CP110 exists in large
(approximately 300 kDa to 3 MDa) complexes that
contain both centrin and CaM.
Analyses detected a single conserved calmodulin
(CaM) homologue, 3 distinct centrin (CETN)-caltractinlike proteins.
Centrin protein is an ubiquitously expressed
cytoskeletal component
Cen-2 binds calcium and magnesium and that calcium
modulates HsCen-2 structure as well as XP.
Centrins
A multi-gene family (Cetn1, Cetn2, and Cetn3) encodes the
calcium-binding protein, centrin, in the mouse. Structurally,
Cetn2 consists of five exons and four introns, and contains
a classical TATA-less promoter. Cetn2 has two alternate
transcription start sites, and a single length 3' untranslated
region. Fluorescence in situ hybridization
demonstrates that Cetn2 is an X-linked gene whose
alleles replicate asynchronously during S-phase.
Cetn2 encodes a 172 amino acid protein, with a predicted
molecular mass of 19,795 Da. Northern blot analysis
indicates that Cetn2 is ubiquitously expressed in the
tissues of adult mice. RT-PCR shows that Cetn2 and
Cetn3, but not Cetn1, are expressed in NIH 3T3 cells.
Immunofluorescence microscopy demonstrates that mouse
centrin 2 protein localizes to the region immediately
surrounding the centrioles in the centrosome of NIH 3T3
cells.
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE IN
MAMMARY CANCER
Without the correct number of centrosomes, human cells could
not divide normally, resulting in a gain or loss of chromosomes
within the genome. What happens when the centrosome
malfunctions is a matter of dispute.
Normal mitotic spindle contains two centrosomes (in orange)
whereas multipolar spindle from tumor cell has four centrosomes.
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE IN MAMMARY
CANCER
Major regulators of centrosomes: the complex Cyclin-
dependent/CDK molecules, (which also are major regulators of
mitosis).
The Nek2 protein kinase, (which is the most closely related vertebrate
protein by sequence to the essential mitotic regulator NIMA of
Aspergillus nidulans).
SCF ubiquitin ligases, composed of three major subunits, Skp1, Cul1,
and one of many F box proteins (Fbps).
Pin1 regulates centrosome duplication, and its overexpression induces
centrosome amplification, chromosome instability, and oncogenesis.
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE
IN MAMMARY CANCER
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE IN MAMMARY
CANCER
The first stage of mitosis is known as prophase. During
prophase, major changes also occur in the cytoplasm, including
disassembly of the cytoskeleton components based on tubulin
(cytoplasmic microtubules). The tubulin contribute to form the
main component of the mitotic apparatus, the mitotic spindle,
which is bounded by the centrosomes.
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE IN MAMMARY
CANCER
Centrins
Centrins: calcium binding proteins from the EF-hand
superfamily implicated in various cellular functions, such as
centrosome duplication and DNA repair.
In some studies using RTPCR and Northern blot analysis
demonstrate that Cetn1 expression is limited exclusively to
the testis in adult male mice.
Centrin/Cdc31p is a Ca2+-binding protein related to
calmodulin found in the MTOC of diverse organisms. In yeast,
Cdc31p localizes to the SPB with Kar1p and is required for
SPB duplication. Recent findings suggest that centrin also
functions elsewhere in the cell
Immunoelectron microscopic localization of centrin 3 in a mouse
photoreceptor cell and schematic illustrations of the centrin isoform
localization in photoreceptor cells and non-photoreceptor cells
Giessl, A. et al. J. Biol. Chem. 2004;279:51472-51481
Expression analysis of centrin isoforms in mouse retina by RT-PCR
Giessl, A. et al. J. Biol. Chem. 2004;279:51472-51481
CENTROSOME AMPLIFICATION AND POSSIBLE ROLE IN BREAST CANCER
Tumour formations arise as a consequence of alterations in the control
of cell proliferation as well as with disorders in interactions between
cells and their environment that result in invasion and metastasis.
Genetic alterations of several proto-oncogenes and tumor-
suppressor genes (e.g. APC/MCC, RAS, DCC, p53 mutations and/or
allelic losses, hyperexpression of c-MYC and RB genes), as well as
other genomic alterations, appear at characteristic stages of tumor
development and are observed in most neoplasms.
Ras and MYC in breast cancer: It is known that expression of
mammalian proteins that inactivate the tumor suppressors Rb and p53
in conjunction with the oncoproteins Ras and Myc and the telomerase
subunit hTERT is sufficient to drive a number of normal human somatic
cells to a tumorigenic fate. This provides a blueprint of the events that
lead to human cancer, allowing different cancers to be genetically
modeled from normal human cells.
• THE IDENTIFICATION OF PROMOTERS AND THEIR
REGULATORY ELEMENTS
Subcellular localization of centrin isoforms in the mouse
retina
Giessl, A. et al. J. Biol. Chem. 2004;279:51472-51481
A PANDORA BOX
Another class of cancer-promoting genes:
Mutations arise from an unrepaired error in DNA. So any gene whose
product participates in DNA repair probably can also behave as an oncogene
when mutated.
Example: ATM. ATM (="ataxia telangiectasia mutated") gets its name from a
human disease of that name, whose patients — among other things — are at
increased risk of cancer. The ATM protein is also involved in detecting DNA
damage and interrupting the cell cycle when damage is found.
It is estimated that fully 1% of the 25,000 or so genes in the human genome
are proto-oncogenes.
Tumor-Suppressor Genes: The products of some genes inhibit mitosis.
These genes are called tumor suppressor genes.
BASIC CONCEPT IN CELL CYCLE REGULATION, AND
RAS, MYC
Many of these involve kinases — enzymes that attach phosphate
groups to other proteins. Examples: the proteins encoded by SRC,
RAF, ABL, and the fusion protein encoded by BCR/ABL.
Molecules that turn on kinases. Example: RAS, which activates RAF.
In most cases, phosphorylation activates the protein and eventually
transfers the signal into the nucleus.
Here phosphorylation activates transcription factors that bind to
promoters and enhancers in DNA, turning on their associated genes.
Examples: AP-1, a heterodimer of the proteins encoded by jun and
fos.
Some of the genes turned on by these transcription factors encode
other transcription factors. Example: myc.
ONCOGENE PUZZLE
An oncogene is a gene that when mutated or expressed at
abnormally-high levels contributes to converting a normal cell
into a cancer cell. Cancer cells are cells that are engaged in
uncontrolled mitosis.
Midgestation embryos were infected with replication-defective
retroviral vectors that either transduced the myc oncogene, the
ras oncogene, or both oncogenes simultaneously. The myc
virus induced tumors in diverse organs at a very low frequency
and with a long latency period, while 20% of the mice derived
from embryos infected with the ras virus developed tumors in
the skin with a latency of 4-8 weeks. In contrast, infection of
embryos with the ras/myc double oncogene virus resulted in
27% of the animals developing rapidly growing and malignant
tumors in a great variety of tissues after a median latency period
of 2-3 weeks. All tumors were of monoclonal.
ENSURING FURTHER INTERDEPARTAMENTAL
COOPERATION AND EVEN CLINICAL TRIALS
FUNDING
Proliferative markers:
S-phase fraction (SPF).
Thymidine labeling index.
IHC analysis using antibodies directed against proliferating-cell nuclear
antigens such as ki-67, and proliferating-cell nuclear antigen.
Assessment of Proliferative markers and centrosome amplification,
predictors and prognostic factors:
Systemic therapy response and testing proliferating markers.
Tumor size, lymph node status, prognosis, node status, histopathological
grade, risk of death, risk of survival.
Adding more variables to test, to make a more valuable models for
humans.
Estrogen and progesterone receptors, and the above factors. Herb2/neu
proto-oncogenes
OMCOGENES AS PART OF A PUZZLE
No single oncogene can, by itself, cause cancer. It can, however,
increase the rate of mitosis of the cell in which it finds itself. Dividing
cells are at increased risk of acquiring mutations, so a clone of
actively dividing cells can yield subclones of cells with a second,
third, etc. oncogene. When a clone loses all control over its mitosis
it is well on its way to developing into a cancer.
WHAT IS THE RESULT OF WHAT?
Epidemiological findings suggest that the consequences of a given
oncogenic stimulus vary depending upon the developmental state of
the target tissue at the time of exposure. This is particularly evident in
the mammary gland, where both age at exposure to a carcinogenic
stimulus and the timing of a first full-term pregnancy can markedly alter
the risk of developing breast cancer. Analogous to this, the biological
consequences of activating oncogenes, such as MYC.
To test this hypothesis directly, we have used a doxycycline-inducible
transgenic mouse model to overexpress MYC during different stages
of mammary gland development.
Generation of the MMTV-rtTA (MTB) transactivator line and the TetO-
MYC (TOM) responder line has been previously described (D'Cruz et al.,
2001 ; Gunther et al., 2002 ). The pTetO-MYC expression vector was
generated by cloning exons 2 and 3 of human MYC from pSV7Humyc
(Murphy et al., 1986 ) into pTet-Splice. Bitransgenic MTB/TOM and
littermate MTB control mice were administered doxycycline (2.0 mg/ml)
in their drinking water to induce expression of the MYC transgene.
EXPERIMENTAL DESIGN EMPHATIZING ON
CENTROSOME AMPLIFICATION
“c-MYC induces mammary tumorigenesis by means of a preferred
pathway involving spontaneous Kras2 mutations:.
'Cruz CM, Gunther EJ, Boxer RB, Hartman JL, Sintasath L, Moody SE,
Cox JD, Ha SI, Belka GK, Golant A, Cardiff RD, Chodosh LA.
Deregulated estrogen receptor alpha expression in mammary epithelial
cells of transgenic mice results in the development of ductal carcinoma
in situ. Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, Chodosh
LA, Flaws JA, Furth PA.
FASEB J. 2002 Mar;16(3):283-92.Related Articles, Links
A novel doxycycline-inducible system for the transgenic analysis of
mammary gland biology.
Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB,
Chodosh LA.
Profile
In te n s i ty
250
200
150
100
50
0
0
Ch2-T1
Ch3-T1
5
Ch2-T2
10
15
20
25
Di s ta n c e (µm )
30
35
40
45
Zdfh9 mice after doxycycline
Profile
In te n s i ty
250
200
150
100
50
0
0
Ch2-T1
20
Ch3-T1
40
60
Di s ta n c e (µ m )
80
100
120
Ch2-T2
Difference in proliferation
Profile
In te n s i ty
250
200
150
100
50
0
0
Ch2-T1
Ch3-T1
10
Ch2-T2
20
30
40
Di s ta n c e (µ m )
50
60
70
Troubleshooting: gamma-tubulin
E-cadh and
Pericentrim
Overlapping
patterns.
Troubleshooting:
Dapi
Pericentrin is ubiquitously
Located, both inside and
outside the nuclei
Zdfhn9
0
50
100
150
200
x 250
300
350
400
Intensity
450
200
100
500
0
0
Profile
In te n s i ty
250
200
150
100
50
0
0
Ch2-T1
Ch3-T1
20
Ch2-T2
40
60
Di s ta n c e (µ m )
80
100
120
100
200
300
y
400
500
600
Antibodies mapping on the tumor, not easy task
Best combination:
gamma-tubulin, and Dapi
Ki 67-Dapi-pericentrim
Dapi-E-cad-Pericentin
A solution may be imagen analysis (D-E-P)
Dapi-E-cad-Pericentrin spontaneous tumor
A b s o l u t e F re q u e n c y Ch 2 -T 1
8000
6000
4000
2000
0
0
50
100
150
200
250
150
200
250
150
200
250
In te n s i ty
M a x i m u m f re q u e n c y i s 8 6 4 8 a t I n t e n s i t y = 2 5 5 .
V a l u e s f o u n d f ro m M i n i m u m = 1 5
to M a x i m u m = 2 5 5 .
A b s o l u t e F re q u e n c y Ch 3 -T 1
6000
4000
2000
0
0
50
100
In te n s i ty
M a x i m u m f re q u e n c y i s 6 1 0 4 a t I n t e n s i t y = 1 7 .
V a l u e s f o u n d f ro m M i n i m u m = 0
to M a x i m u m = 2 5 5 .
A b s o l u t e F re q u e n c y Ch 2 -T 2
3000
2000
1000
0
0
50
100
In te n s i ty
M a x i m u m f re q u e n c y i s 3 1 6 5 a t I n t e n s i t y = 5 7 .
V a l u e s f o u n d f ro m M i n i m u m = 1 5
to M a x i m u m = 2 5 5 .
Ab s o l u t e F re q u e n c y Ch 2 -T 1
8000
6000
4000
2000
0
0
50
100
150
200
250
150
200
250
150
200
250
In te n s i ty
M a x i m u m f re q u e n c y i s 8 6 4 8 a t I n t e n s i t y = 2 5 5 .
Va l u e s f o u n d f ro m M i n i m u m = 1 5
to M a x i m u m = 2 5 5 .
Ab s o l u t e F re q u e n c y Ch 3 -T 1
6000
4000
2000
0
0
50
100
In te n s i ty
M a x i m u m f re q u e n c y i s 6 1 0 4 a t I n t e n s i t y = 1 7 .
Va l u e s f o u n d f ro m M i n i m u m = 0
to M a x i m u m = 2 5 5 .
Ab s o l u t e F re q u e n c y Ch 2 -T 2
3000
2000
1000
0
0
50
100
In te n s i ty
M a x i m u m f re q u e n c y i s 3 1 6 5 a t I n t e n s i t y = 5 7 .
Va l u e s f o u n d f ro m M i n i m u m = 1 5
to M a x i m u m = 2 5 5 .
500
500
450
450
400
400
350
350
300
300
Intensity
250 y
250 y
200
200
150
150
100
250
200
150
100
50
0
50
0
0
50
100
150
200
250
x
300
350
400
450
500
Intensity
100
250
200
150
100
50
0
50
0
0
50
100
150
200
250
x
300
350
400
450
500
500
450
400
350
300
y 250
200
150
Intensity
100
200
50
100
0
0
0
50
100
150
200
250
x
300
350
400
450
500
Mohs?
Intensity
250
150
500
400
50
0
300
50
100 150
200 250
300 350
400 450
x
500
0
50
200
y
100
Gamma-tubulin basal
expression
DAPI
GAMATUBULIN
DAPI/ GAMATUBULIN
DAPI/ GAMATUBULIN
EXPERIMENTAL DESIGN EMPHATIZING ON
CENTROSOME AMPLIFICATION (CA)
AIMS: To develop mice in which ras and Myc are conditionally induced in
mammary gland and to be able to observe centrosome (mostly CA) dynamics
in vivo. To Correlate or not the protective roles of CDk2 and CDk4 (if ablated)
on the presence of CA and therefore in tumorigenesis. Do the
phosphorylation of Nucleophosmin (NPM) and Metallopanstimulin (Mps01)
play a role in this regulatory puzzle?
A. Cross centrin-GFP mice generated in our lab with transgenic mice that contains the
tretracycline-myc-Ras inducible system. Be able to determine the efficient expression
of Ras and myc and the non involvement of centrosome abnormalities in the control
mices.
B. Does CA play a role in the tumorigenis process?
C. Do CA precede induction of RAS-Myc?,
D. Is any clinical correlation among tumor stages and CA or is non correlation?
E. To Correlate or not the protective roles of CDk2 and CDk4 (if ablated) on the
presence of CA and therefore in tumorigenesis.
Clever system: Unfortunately, constitutive transgenic mouse models that rely on
mammary-specific promoters to control transgene expression have limited utility for
studying the effect of developmental events on breast cancer risk since the hormonal
signals governing these events also markedly influence transgene expression levels. A
novel transgenic mouse system is described that uses the MMTV-LTR to drive
expression of the reverse tetracycline-dependent transactivator rtTA
.
EXPERIMENTAL DESIGN EMPHATIZING ON
CENTROSOME AMPLIFICATION (CA)
FUNDAMENTAL KNOWLEDGE AND BACKGROUND:
- Nucleophosmin (NPM) is a multifunctional protein frequently overexpressed in
actively proliferating cells and Metallopanstimulin (Mps-1).
- Myc induces CA on mouse embryonic fibroblast.
- Centrin 2 (to evaluate centriole dynamics). Centrin 2 is require for
centrosome duplication.
- Addition of doxycyclin results in expression o the reporter gene LacZ in
isolated mammary epithelia cells.
-By crossing transgenic mices containing the MMTV-RTA to transgenic mice
containing any gene cloned downstream of the tet operator (tet-O) and
administrating the doxycycline = transcription of Myc, and appearance of 87%
of adenocarcinomas. This tumor model stresses the central role of played by
RAs and Myc.
A novel doxycycline-inducible system for the
transgenic analysis of mammary gland biology.
Transgene expression in MTB/TZA bitransgenic mice. Expression of
rtTA in the mammary glands of MMTV-rtTA-pA (MTB) mice.
Northern hybridization analysis of total RNA from the mammary
glands of 5-wk-old nulliparous transgenic and wild-type female mice
for rtTA expression. The presence or absence of the transgene is
indicated. Mice were administered 2 mg/ml doxycycline in drinking
water for 72 h. B) Doxycycline-inducible expression of a ßgalactosidase reporter gene in bitransgenic mice. Virgin female mice
(6-wk-old) of the indicated genotypes were either left untreated or
administered 2 mg/ml doxycycline in drinking water for 72 h. Mammary
gland extracts were prepared and assayed for ß-galactosidase activity.
C) Homogeneous, mammary epithelial-specific ß-galactosidase
activity in doxycycline-treated MTB/TZA mice: 6-wk-old virgin female
bitransgenic mice were either left untreated (left panels) or induced
with doxycycline. Note homogeneous staining of mammary epithelium,
were whole mounted or embedded in OCT and sectioned before IHC
staining for ß-galactosidase activity.
A novel doxycycline-inducible system for the
transgenic analysis of mammary gland biology.
EXPERIMENTAL DESIGN CENTROSOME AMPLIFICATION
TECHNIQUES and experimental tools:
Transgenic mice (deregulation on oncogenes e.g. MMTV-ras/myc),
morphological analysis, colony husbandry crossings,
Immunohistochemistry and TUNEL analysis, Northern hybridization,
Western analysis, Oligonucleotide microarray hybridization, Mammary
Gland Whole Mounts. Green fluorescent protein (GFP) (allows direct
visualization of many proteins). Knockout mices (CDks).
Expected outcome: pre-tumor appearance can be correlated with CA
and the possible role of RAS and Myc in CA.
Do RAS and Myc control any known pathway in CA?
Is non correlation with CA, cancer?
EXPERIMENTAL DESIGN EMPHATIZING ON
CENTROSOME AMPLIFICATION (CA)
MMTV-rtTA
Centrin-GFP
Tet 0 RAS
Tet 0myc
X
rtTA -/+
Tet 0 RAS
Tet 0myc
3 D culture
Frozen sections
100 epithelial cells per
slide for CA detection
Verify for
northern
EXPERIMENTAL DESIGN EMPHATIZING ON
CENTROSOME AMPLIFICATION (CA)
P-53, ErB pathway, NPM, SRC, RAF, ABL
Pericentrim, gamma tubulin, crest, k167, and Ecadherin
Features: FVB embryos have pronuclei that are
easily manipulated for transgenic microinjection
projects. This, coupled with high reproductive
performance, makes the FVB ideally suited for
transgenic studies.
OVERCOMING PITFALLS
Genotyping of targeted transgenics and point mutations is
performed by PCR or SNP analysis as appropriate.
Zygosity of insertional transgenics can be determined by QPCR.
(Transgene-Specific Testing: This Quantitative PCR (QPCR) assay
allows determination of two-fold difference in copy number between
hemizygote and homozygote transgenic rodents. In-house zygosity
assay development is rigorously optimized and validated).
Background strain characterization ensures that your model is
on the expected strain.
MAX-BAXSM speed congenics accelerates the development of a
congenic knockout or transgenic mouse or rat line.
Molecular phenotyping can determine transgene copy number
OVERCOMING PITFALLS
Genetic Testing Services: Expression Testing
How many generations to be sure the trangenes and or mutations are
stable?
Quantitative reverse transcriptase PCR (RT-QPCR) methods are used
to quantify the steady-state level of mRNA expression from a transgene or
knockout construct within an animal model. Expression testing protocols
require in-house assay development and are rigorously optimized and
validated.
Quality of the Transgenic Mice that we are using:
Transgenic mice are constructed by injecting cloned DNA into fertilized mouse
eggs; those eggs that survive are then implanted in foster females to develop to
term. The gestation period of the mouse is 19-21 days. Pups are ready for
weaning at 3-4 weeks of age, and reach sexual maturity at 6 weeks (females) to
8 weeks (males). The minimum elapsed time between injection of the construct
and readiness for breeding of the transgenic founders is 9 to 11 weeks. For
most experimental purposes researchers will want to use offspring of a
transgenic animal, rather than the founder animal itself.
Tubulin isotype distribution may play a role in the
development of anti-cancer anti-tubulin drug resistance as
well as in drug efficacy and specificity cells resistant to
combretastatin A-4 (CA4), paclitaxel or vinblastine. Western
analysis demonstrated alterations in total beta-tubulin and
classes I, III and IV tubulin isotypes among the resistant H460
cell lines. Class III beta-tubulin was significantly altered in all
resistant cell lines.
Centrosome amplification has been proposed to contribute to
the development of aneuploidy and genome instability. Here,
we show that Ataxia-Telangiectasia Mutated (ATM) is localized
to the centrosome and co-purified with gamma-tubulin. The
importance of ATM in centrosome duplication is
demonstrated in Atm-deficient primary mouse embryonic
fibroblasts that display centrosome amplification.
TRANSGENIC MICE AND KNOCK-OUT-IN MICE (VERIFICATION TWICE YEARLY
Creator, and all the information to be provide to the Department administrators.
Patent. The Department administrators obtain a copy from NIH, OTT. http://www.ssti.org/Digest/Tables/120100t.htm
Institutional Material agreement transfer obtain for specific project #.....
Verification for DAR that the breeding and the genotypes of the mice are use for approved proposal
Primers, do these belong to the vectors or to the gene of interest.
Mouse strain. How many generations have been keep on track.
http://www.nih.gov/science/models/mouse/sharing/5.html. Other
licensing:http://www.pharmcast.com/PatentToSubWeb/Licensing/Licensing.htm#Listing
1.
2.
Spontaneous known tumor type and differentiation….
Expected tumor type….
Permit use for the double and triple crossing with transgenic and or knock-out-in mice. Are the results expected to
be same in the experiments using the same crossing as control?
Known hormonal, age, problems with the vector, with the transgenic and or knockouts-in mice.
1: J Neuropathol Exp Neurol. 2006 May;65(5):465-77
Cellular gamma-tubulin was detected in both soluble and
insoluble (nocodazole-resistant) fractions of glioblastoma
cells.
Divergent localizations of gamma-tubulin and pericentrin
suggest a differential distribution of these 2 centrosomeassociated proteins in glioblastoma cell lines.
“Our results indicate that overexpression
and ectopic cellular distribution of gamma-tubulin
in astrocytic gliomas may be significant in the
context of centrosome protein amplification and
may be lin”ked to tumor progression and anaplastic
potential”.
…Ran is tightly associated with the centrosome
Throughout the cell cycle. Ran interaction with
the centrosome is mediated by the centrosomal
matrix A kinase anchoring protein (AKAP450).
… We also demonstrate that centrosomal proteins such
as centrin and pericentrin, but not gamma-tubulin,
AKAP450, or ninein, undertake a nucleocytoplasmic
exchange as they concentrate in the nucleus upon
export inhibition by leptomycin B.
…. pericentrin/kendrin-ninein.
1: Mol Biol Cell. 2003 Oct;14(10):4260-71. Epub 2003 Jul 11
Formation of the apoptotic microtubule array in mid-to-late apoptosis
Mooss, D. K. et al. J Cell Sci 2006;119:2362-2374
Effects of apoptosis on centrosome integrity
Moss, D. K. et al. J Cell Sci 2006;119:2362-2374
Fluorescence microscopy of microtubule and actin distribution in apoptotic A431 cells
Moss, D. K. et al. J Cell Sci 2006;119:2362-2374
Guy Keryer mbc on July 11, 2003
EXPERIMENTAL DESIGN EMPHATIZING
ON CENTROSOME AMPLIFICATION (CA)
Know natural pitfalls to overcome:
Multiples. Normal developmental events such as
puberty, pregnancy, and parity influence the
susceptibility of the mammary gland to tumorigenesis in
both humans and rodent model systems.
Phosphoisotopes antibodies?