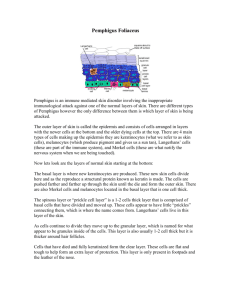
Received: 4 January 2019 Revised: 7 August 2019 Accepted: 12 August 2019 DOI: 10.1111/cup.13565 ORIGINAL ARTICLE Membrane attack complex (C5b-9 complex or Mac), is strongly present in lesional skin from patients with endemic pemphigus foliaceus in El Bagre, Colombia Ana Maria Abreu-Velez1 | Yulieth A. Upegui-Zapata2 | Carlos A. Valencia-Yepes3 | Eduardo Upegui-Quiceno4 | Natalia-Regina Mesa-Herrera5 | Alejandra M. Jiménez-Echavarria4 | César D. N. Pulido6 | Bruce R. Smoller7,8 | Michael S. Howard1 1 Georgia Dermatopathology Associates, Atlanta, Georgia 2 School of Medicine, Medical Research Institute, University of Antioquia, Medellin, Colombia 3 Abstract Background: El Bagre endemic pemphigus foliaceus (El Bagre-EPF) is a new variant of endemic pemphigus foliaceus present in the El Bagre area of Colombia, South Department of Education, University of Antioquia, Medellin, Colombia America. Here, we investigate the presence of complement/C5-b9 in lesional skin of 4 patients and matched controls from the endemic area. We also aim to compare the Programa de Estudio y Control de Enfermedades Tropicales Group, University of Antioquia, Medellin, Colombia 5 Biogemol Institute and Department of Physiology and Biochemistry, School of Medicine University of Antioquia, Medellin, Colombia 6 Department of Cardiology, CES University, Medellin, Colombia 7 Department of Pathology and Laboratory Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York 8 Department of Dermatology, University of Rochester School of Medicine and Dentistry, Rochester, New York patient's autoantibody levels using indirect immunofluorescent titers (IIF) and correlate with the lesional presence of complement/C5b-9. Methods: A case-control study was carried out by testing for the presence of complement/C5b-9 in lesional skin in 43 patients affected by El Bagre-EPF, as well as 43 matched, healthy controls from the endemic area. Skin biopsies were obtained and evaluated via hematoxylin and eosin staining, and immunohistochemistry. Results: The presence of complement/C5b-9 was observed in all cases of the patients affected by El Bagre-EPF and was not observed in the controls from the endemic area (P < 0.001). The patients' autoantibody titers utilizing IIF for IgG and IgM showed correlation between higher autoantibody titers and stronger intensity of Correspondence Ana Maria Abreu-Velez, MD, PhD, Georgia Dermatopathology Associates, 1534 North Decatur Road, NE, Suite 206, Atlanta, GA 30307-1000. Email: abreuvelez@yahoo.com staining with complement/C5-b9 staining (P < 0.001). Funding information Embassy of Japan in Colombia; Georgia Dermatopathology Associates, Atlanta, GA; Hospital Nuestra Señora del Carmen, El Bagre; Mineros SA; Municipality of El Bagre, Antioquia, Colombia C5-B9, endemic pemphigus foliaceus in El Bagre, MAC complex Conclusion: Patients affected by El Bagre-EPF have lesional deposition of complement/ C5b, which correlates with disease severity and previously established serologies. KEYWORDS Abbreviations: BMZ, basement membrane zone; CS, complement system; C5b-9 complex or MAC, membrane attack complex; DAPI, 4',6-diamidino-2-phenylindole; DIF, IIF, direct and indirect immunofluorescence; EPF, endemic pemphigus foliaceus; El Bagre-EPF, endemic pemphigus foliaceus in El Bagre; FITC, fluorescein isothiocyanate; FS, fogo selvagem; H&E, hematoxylin and eosin; ICS, intercellular stain between keratinocytes; PF, pemphigus foliaceus; SNPs, single-nucleotide polymorphisms. © 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd. J Cutan Pathol. 2019;1–5. wileyonlinelibrary.com/journal/cup 1 2 ABREU-VELEZ ET AL. conjugated monoclonal antibodies to human total IgG or IgG4, as 1 | I N T RO D UC T I O N described elsewhere9-11; (d) and test positive by immunoblotting for From the first documentation of the autoimmune nature of pemphi- reactivity against desmoglein 1 (Dsg1),9,10 (e) and for plakin molecules gus (including pemphigus foliaceus [PF] and its variants including including envoplakin, periplakin, and/or desmoplakins I-II. Additional endemic pemphigus foliaceus [EPF]), the role of autoantibodies and criteria included (f) that each patient's serum must immunoprecipitate the complement system (CS) has been recognized as pathogenic in a concanavalin A affinity-purified bovine tryptic 45 kDa fragment of 1-6 Multiple authors have described Dsg1,12 and (g) each patient's serum had to yield a positive result the association of the CS and its fixation by pemphigus antibodies, using an ELISA test when screening for autoantibodies to PF anti- documenting the role that the CS plays in pemphigus.1-6 In pemphi- gens.13 In this study, one biopsy specimen was taken in each case; half gus, as well as in Brazilian EPF (fogo selvagem), granular comp- to be used for H&E and immunohistochemistry (IHC) stains, and half lement/C3 deposits along intercellular spaces of the epidermis (ICS) for immunofluorescence examination. lesional skin and in cell cultures. combined with IgG autoantibodies were documented as a diagnostic hallmark.7,8 Here, we aim to determine the presence of the membrane 2.1 | H&E and IHC attack complex/MAC (complement/C5b-9 complex), in lesional skin using a case-control study in patients affected by a new variant of We performed H&E and IHC stains as previously described.14 All sam- endemic pemphigus foliaceus (El Bagre-EPF) in El Bagre, Colombia, ples were run with positive and negative controls. The interpretation South America. This variant of EPF, in contradistinction to fogo of IHC was based on overall staining intensity15: intensity (0: no selvagem, affects men more than women, and is not present in people immunoexpression; 1+: weak immunoexpression; 2+: moderate immu- younger than 20 years; further, in about one third of the patients, noexpression; and 3+: strong immunoexpression first developed by there are autoantibodies directed not only to the skin but to cell junc- McCarty et al, and termed an H&E score. For our IHC staining we uti- 9-15 lized a Leica (Buffalo Grove, IL) staining system. Specifically, for pri- The study rationale was based on the fact that we recently discovered mary staining we utilized a Bond Max platform autostainer with bond new intra-cytoplasmic and intranuclear autoantigens. Such data imply polymer refined detection DS9800, a horseradish peroxidase linker the opening of the cell membrane, such as may happen through the polymer, and DAB chromogen (brown staining). Positive and negative ring structure formed by a complement/C9 pore in the membrane, all- controls were consistently included for study. For IHC, we utilized owing free diffusion of molecules into and out of the cells. antibodies for complement/C5b-9 at the dilution of 1:50, without tions in multiple organs of the body, causing systemic anomalies. antigen retrieval (Dako; Agilent Technologies, Santa Clara, CA). 2 | MATERIALS AND METHODS 2.2 | Statement on ethics A case-control study was done by searching for the existence of complement/C5-b9 in lesional skin in patients, and controls appropriately matched from the endemic area. We tested 43 patients affected by El Bagre-EPF and 43 controls matched by age, sex, demographics, comorbidities, work activities, weight, exposure to chemicals, socioeconomic status and income, and food intake. About one-third of El Bagre-EPF patients had a Senear-Usher-like disease, as documented previously.9,10 The 43 controls from the endemic A human quality assurance review board approved the studies at the Hospital Nuestra Señora del Carmen in El Bagre and all participants provided signed consent. The studies have been approved by the appropriate institutional and/or national research ethics committees, and performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. area were healthy individuals without skin disease. As internal controls, we also tested skin samples from healthy patient breast reduc- 3 | RESULTS tions, five patients with cutaneous lupus, five with bullous pemphigoid (BP), five with dermatitis herpetiformis (DH), and five Figure 1A shows a typical clinical hyperpigmented form in a patient with pemphigus vulgaris (PV). affected by El Bagre-EPF. Figure 1B shows a typical H&E stained Skin biopsy specimens from the patients and controls were evalu- biopsy, showing small intra-corneal blisters, as well as acantholysis in ated by hematoxylin and eosin (H&E), direct and indirect immunofluo- the epidermis. In Figures 1C,D, we show some of the patterns of rescence (DIF, IIF), and immunoblotting, immunoprecipitation, and positivity seen by IHC staining using the complement/C5b-9 enzyme-linked immunosorbent assay (ELISA). Only patients meeting antibody. the following diagnostic criteria for El Bagre-EPF were included; spe- The patients with a larger percentage of compromised skin cifically, they had to: (a) display clinical and epidemiological features (according to the Lund and Browder skin burn area chart)16 showed described for the disease; (b) live in the endemic area;9,10 (c) have higher titers of serum autoantibodies, as well as stronger reaction to the serum displaying intercellular staining (ICS) between epidermal complement/C5-b9 antibody. The data parallel the clinical forms of pem- keratinocytes and the basement membrane zone (BMZ) of the skin phigus (according to Viera's clinical classification for fogo selvagem).17 demonstrated via either DIF or IIF using fluorescein isothiocyanate (a) The clinically inactive form (no apparent clinical compromise, but still 3 ABREU-VELEZ ET AL. F I G U R E 1 A, A clinically hyperpigmented form in a patient affected by El Bagre-EPF with hyperkeratotic plaques on the back (white arrow). B, An hematoxylin and eosin (H&E) stain of a patient affected by El Bagre-EPF showing the epidermal edema (black arrow) and a small subcorneal blister (red arrow; ×400). C, IHC stain using antibody to C5-b9 shows positive staining (indicated in fuschia) in the corneal layer (red arrow; +++), BMZ (green arrow; +++), and the extracellular matrix (black arrow; ++; ×100). D, AntiC5-b9 staining at higher magnification highlighting positive staining in the corneal layer (black arrow) and the BMZ (green arrow; ×400). E, Positive antiC5-b9 staining on extracellular matrix fibers and/or mesenchymal-endothelial cell junctions (black arrows; ++), sebaceous gland basement membrane (red arrow; ++), and eccrine glands (blue arrow; +++) in El Bagre-EPF. F, A representative section of normal skin showing negative anti-C5-b9 immunohistochemical staining (×200) positive via our DIF with ICS [positive staining +] and serum antibody antibody (as well as presence in specific parts of the epidermis, the der- titers 1:20 to 1:40. (b) The localized, prurigoid, and hyperpigmented mis, and the skin appendages with the intensity of positivity). forms (9%-24% of the skin involved; our positive staining for complement/C5-b9 was ++, present in the locations presented in Table 1). (c) The Senear-Usher and generalized forms including erythrodermic, bullous exfoliative, and hyperkeratotic (from 24%-100% skin involvement; our complement/C5-b9 staining was +++, and our serum titers of autoantibodies ran between 1:320 and 1:640). 3.1 | Assessing the autoantibody levels in relation to disease severity and therapeutic response in El BagreEPF pemphigus patients The more active clinical cases displayed not only intercellular staining In Table 1, we show the results of our IHC staining in El Bagre-EPF, between the keratinocytes (ICS), but also to the BMZ, to dermal neu- with controls from the endemic area, as well as from patients with other rovascular bundles, to dermal skin appendages and their cell junctions, autoimmune skin diseases. The table displays the localization of immuno- to neuroreceptors, and to mesenchymal-endothelial dermal cell reactivity, and the strength of the staining using the complement/C5b-9 junctions. 4 ABREU-VELEZ ET AL. T A B L E 1 IHC staining showing the number of positive stain in the skin and its appendages sites, correlation with skin severity affecting percentage of skin accordingly with the scale use for burns, IIF serologic titers of autoantibodies in this study using C5-B9 antibody Number of El Bagre-EPF cases Strength of staining Positivity to the skin sites and its appendices Controls from the endemic area Lupus BP DH PV 5/43 (+++) Intracorneal stain 0/43 0/5 0/5 4/5 0/5 43/43 (+++) Intercellular stain between the keratinocytes 0/43 0/5 0/5 0/5 5/5 25/43 (++) BMZ 0/43 5/5 5/5 Below the sub epidermal blister mainly in the papillary dermis (5/5) 0/5 43/43 (++) Neurovascular bundles 0/43 3/5 3/5 3/4 3/5 23/43 (++) Sebaceous gland basal membrane 0/43 3/5 0/5 0/5 0/5 31/43 (++) Sweat glands and their ductus 0/43 3/5 3/5 0/5 3/5 32/43 (++) Mesenchymal-endothelial cell junctions in the dermal connective tissue 0/43 0/5 4/5 0/5 0/5 34/43 (+++) Dermal spindled cells 0/43 0/5 2/5 0/5 0/5 Abbreviations: BMZ, basement membrane zone; BP, bullous pemphigoid; DH, dermatitis herpetiformis; PV, pemphigus vulgaris. 4 | DISCUSSION pathway, with the contribution of mannose-binding lectin (MBL and MBL-associated proteases).19-22 All three pathways lead to the activation We previously reported that in El Bagre-EPF the immune response in of the complement C5, C6, C7, C8, and C9 proteins, resulting in the acute cases begins with IgG autoantibodies. However, all cases progress sequential assemblies of C5b-7, C5b-8, and C5b-9 on the target cell. This toward chronicity, and often show relapsing episodes. We documented process results in cell membrane pore formation.19-22 The mechanism of that the immune response eventually becomes polyclonal, displaying pore formation contrasts with pore formation via proteins such as perforin autoreactivity directed toward epidermal keratinocytic intercellular junc- and the cholesterol dependent cytolysins, where it is believed that tions as well as the dermal-epidermal BMZ, and the BMZs of dermal skin pre-pore assembly happens prior to the concurrent release of the trans- appendages. In addition, autoreactivity is noted against neurovascular membrane regions.22 The activation of complement and the subsequent supply cell junctions, and against multiple skin neural receptors.18 Other formation of complement/C5b-9 open channels on cell membranes, immunoglobulins and complement components such as IgM, IgE, IgD, usually leading to cell death. However, when the number of channels complement/C1q, complement/C3c, complement/C4, and kappa and assembled on the surface of nucleated cells is limited, sublytic comple- lambda light chains are seen, in addition to the previously reported IgG.7 ment C5b-9 can induce cell cycle progression by activating signal trans- Often weak ICS staining with IgG is observed in chronic cases. In addition duction pathways and transcription factors, and inhibiting apoptosis.22 to this staining, other immunoglobulins and complement as well as Recently, patients affected by PF including EPF, were evaluated for deposits of fibrinogen and albumin are also seen targeting unique mole- 992 single-nucleotide polymorphisms (SNPs) of 44 CS genes, genotyped cules in other parts of the skin. As reported previously, in about one-third through microarray hybridization in 229 PF patients, and 194 controls.23 of the El Bagre-EPF patients the immune response is directed not only to After excluding SNPs with minor allele frequency they found some gene the skin, but also to several cell-junction and neural receptors in most expression (<1%), out of a Hardy-Weinberg equilibrium in controls or in organs and tissues.18 The patients with a more extended percentage of strong linkage disequilibrium (r2 ≥ 0.8); 201 SNPs remained for logistic compromised skin (according to Lund and Browder skin burn surface regression. The authors also reported increased serum levels of comple- 16 scale) show higher titers of serum autoantibodies as well as stronger reactivity to the complement/C5-b9 antibody. This is indicative of epi- ment C3, and C5-b9 deposition in lesions observed more frequently in the PF and EPF patients in comparison with the normal controls.23 18 Of interest is the correlation we found between positivity of other when the initial antibodies may damage the cell plasma membrane autoimmune blistering and skin diseases such lupus, BP, DH, and PV exposing internal cell molecules to the immune system.19 and the positivity of the staining for complement C5-b9 that perfectly tope spreading and/or exposure to new autoantigens as shown by us The CS contains multiple plasma- and membrane-bound proteins and matched what we had reported on IHC staining with other immuno- receptors working in the vanguard of host defense, killing pathogens, globulins and complement.24 Indeed, our findings suggest that in opsonizing and changing cells and microorganisms, and linking innate these other autoimmune skin diseases, the immune response may also 19-22 immunity to adaptive immune responses. The CS is stimulated in three ways: via the classical pathway, which comprises the complement be more complex than the classically described and currently accepted mechanisms. proteins C1, C2, C3, and C4; the alternative pathway, with the involve- In this study, we confirm previous records that determined that ment of complement C3 and protein factors B, D, and P; and the lectin late components of the complement cascade such as C5-b9 are seen 5 ABREU-VELEZ ET AL. in skin biopsies from PV and PF.25 In El Bagre-EPF, we have noted intracellular and nuclear antigens that are not yet characterized. We hypothesize that this may be the result of the attachment of C5-b9 to the plasma membranes, exposing antigens by either antigen spreading epitopes and or possibly by neoantigen formation during cell membrane damage. We believe this process exposes cell membrane, cytoplasmic and nuclear antigens resulting in the targeting of normally protected antigens by dysregulated (intolerant) lymphocytes. 5 | CO NC LUSIO N This study suggests that C5-b9 deposition measured by IHC staining may be a biomarker for more intense disease activity both clinically and immunologically in patients affected by El Bagre-EPF, and possibly in other autoimmune skin blistering diseases. CONF LICT OF IN TE RE ST None. ORCID Ana Maria Abreu-Velez https://orcid.org/0000-0002-7692-4133 RE FE R ENC E S 1. Cormane RH, Chorzelski TP. Bound complement in the epidermis of patients with pemphigus vulgaris. Dermatologica. 1967;134(6): 463-466. 2. Cormane RH, Chorzelski T. Localization of complement in epithelium by means of immunofluorescence in patients with pemphigus. (preliminary report). Przegl Dermatol. 1967;54(4):432-436. 3. Kawana S, Janson M, Jordon RE. Complement fixation by pemphigus antibody. I. in vitro fixation to organ and tissue culture skin. J Invest Dermatol. 1984;82(5):506-510. 4. Kawana S, Geoghegan WD, Jordon R. Complement fixation by pemphigus antibody. III. Altered epidermal cell membrane integrity mediated by pemphigus antibody and complement. J Invest Dermatol. 1986;86(1):29-33. 5. Krasny SA, Kumar V, Tarfare N, Beutner EH, Chorzelski TP. Intercellular complement C3 binding in normal skin of patients without pemphigus. J Am Acad Dermatol. 1986;14(1):52-58. 6. Xia P, Jordon RE, Geoghegan W. Complement fixation by pemphigus antibody. V. Assembly of the membrane attack complex on cultured human keratinocytes. J Clin Invest. 1988;82(6):1939-1947. 7. dos Reis VM, Cucé LC, Rivitti EA. Anatomopathology and direct and indirect immunofluorescence of lesions of endemic pemphigus foliaceus resistant to corticoid therapy. Rev Inst Med Trop Sao Paulo. 1991;33(2):97-103. 8. Messias-Reason I, Bosco DG, Nisihara RM, Jakobsen LH, PetzlErler ML, Jensenius JC. Circulating levels of mannan-binding lectin (MBL) and MBL-associated serine protease 2 in endemic pemphigus foliaceus. Clin Exp Dermatol. 2008;33(4):495-497. 9. Abreu Velez AM, Hashimoto T, Bollag W, et al. A unique form of endemic pemphigus in northern Colombia. J Am Acad Dermatol. 2003; 49(4):599-608. 10. Abreu Velez AM, Beutner EH, Montoya F, et al. Analyses of autoantigens in a new form of endemic pemphigus foliaceus in Colombia. J Am Acad Dermatol. 2003;49(4):609-614. 11. Hisamatsu Y, Abreu Velez AM, Amagai M, Ogawa MM, Kanzaki T, Hashimoto T. Comparative study of autoantigen profile between Colombian and Brazilian types of endemic pemphigus foliaceus by various biochemical and molecular biological techniques. J Dermatol Sci. 2003;32(1): 33-41. 12. Abreu Velez AM, Javier Patiño P, Montoya F, et al. The tryptic cleavage product of the mature form of the bovine desmoglein 1 ectodomain is one of the antigen moieties immunoprecipitated by all sera from symptomatic patients affected by a new variant of endemic pemphigus. Eur J Dermatol. 2003;13(4):359-366. 13. Abreu-Velez AM, Yepes MM, Patiño PJ, et al. A cost-effective, sensitive and specific enzyme linked immunosorbent assay useful for detecting a heterogeneous antibody population in sera from people suffering a new variant of endemic pemphigus. Arch Dermatol Res. 2004;295(10):434-441. 14. Abreu-Velez AM, Yepes-Naranjo MM, Avila IC, et al. Tissue inhibitor of metalloproteinase 1, matrix metalloproteinase 9, αlpha-1 antitrypsin, metallothionein and urokinase type plasminogen activator receptor in skin biopsies from patients affected by autoimmune blistering diseases. Our Dermatol Online. 2013;4(3):275-280. 15. Vijayashree R, Aruthra P, Ramesh Rao KA. Comparison of manual and automated methods of quantitation of oestrogen/progesterone receptor expression in breast carcinoma. J Clin Diagn Res. 2015;9(3): EC01-EC05. 16. Lund CC, Browder NC. The estimation of areas of burns. Surg Gynecol Obstet. 1944;79:352-358. 17. Viera JP. Consirecoes sobre o penfigo foliaceo no Brasil. Sao Paulo [Different aspects of Brazilian pemphigus foliaceus]. Sao Paulo:EmpresaGraficada RevistadosTribunais; 1948:220. 18. Abreu-Velez AM, Howard MS, Yi H, Florez-Vargas AA. Patients affected by a new variant of endemic pemphigus foliaceus have autoantibodies colocalizing with MYZAP, p0071, desmoplakins 1-2 and ARVCF, causing renal damage. Clin Exp Dermatol. 2018;43(6):692-702. 19. Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321-347. 20. Shin ML, Rus HG, Niculescu FI. Membranes attack by complement: assembly and biology of the terminal complement complexes. In: Lee A, ed. Biomembranes. JAI Press; 1996:123-149. 21. Spicer BA, Law RHP, Caradoc-Davies TT, et al. The first transmembrane region of complement component-9 acts as a brake on its selfassembly. Nat Commun. 2018;9(1):3266. 22. Tegla CA, Cudrici C, Patel S, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol Res. 2011;51(1):45-60. 23. Bumiller-Bini V, Cipolla GA, de Almeida RC, Petzl-Erler ML, Augusto DG, Boldt ABW. Sparking fire under the skin? Answers from the association of complement genes with pemphigus foliaceus. Front Immunol. 2018;9:695. 24. Abreu-Velez AM, Googe PB Jr, Howard MS. Immunohistochemistry versus immunofluorescence in the diagnosis of autoimmune blistering diseases. Our Dermatol Online. 2013;4(suppl. 3):585-595. 25. Kawana S, Geoghegan WD, Jordon RE, Nishiyama S. Deposition of the membrane attack complex of complement in pemphigus vulgaris and pemphigus foliaceus skin. J Invest Dermatol. 1989;92(4):588-592. How to cite this article: Abreu-Velez AM, Upegui-Zapata YA, Valencia-Yepes CA, et al. Membrane attack complex (C5b-9 complex or Mac), is strongly present in lesional skin from patients with endemic pemphigus foliaceus in El Bagre, Colombia. J Cutan Pathol. 2019;1–5. https://doi.org/10.1111/ cup.13565