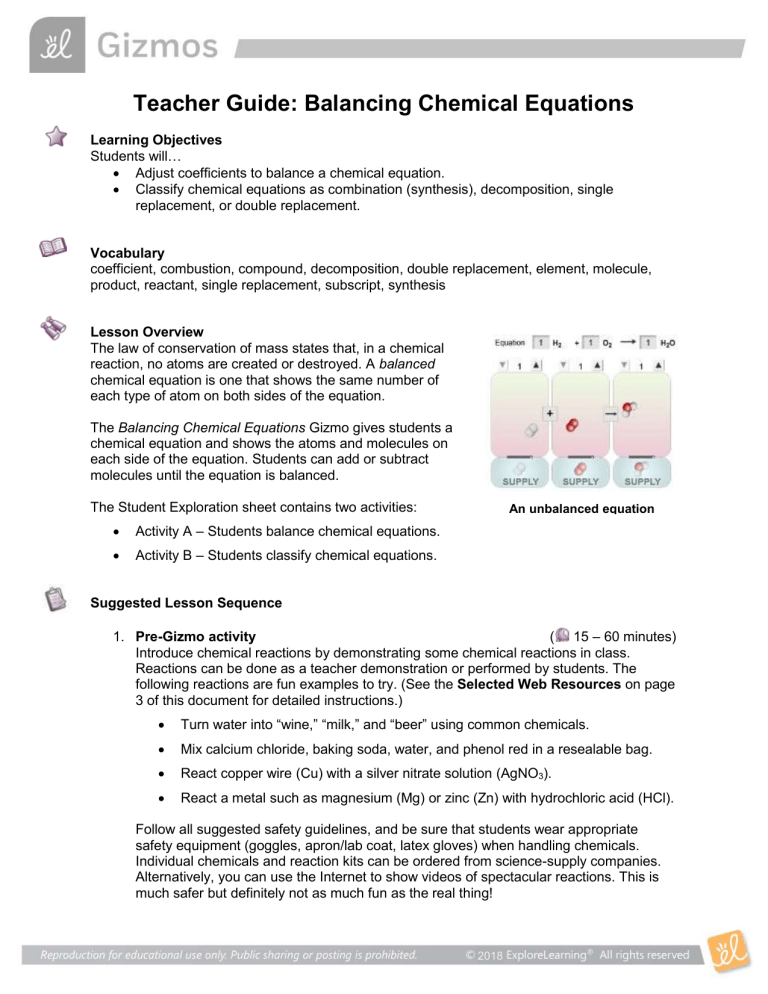
Teacher Guide: Balancing Chemical Equations Learning Objectives Students will… Adjust coefficients to balance a chemical equation. Classify chemical equations as combination (synthesis), decomposition, single replacement, or double replacement. Vocabulary coefficient, combustion, compound, decomposition, double replacement, element, molecule, product, reactant, single replacement, subscript, synthesis Lesson Overview The law of conservation of mass states that, in a chemical reaction, no atoms are created or destroyed. A balanced chemical equation is one that shows the same number of each type of atom on both sides of the equation. The Balancing Chemical Equations Gizmo gives students a chemical equation and shows the atoms and molecules on each side of the equation. Students can add or subtract molecules until the equation is balanced. The Student Exploration sheet contains two activities: Activity A – Students balance chemical equations. Activity B – Students classify chemical equations. An unbalanced equation Suggested Lesson Sequence 1. Pre-Gizmo activity ( 15 – 60 minutes) Introduce chemical reactions by demonstrating some chemical reactions in class. Reactions can be done as a teacher demonstration or performed by students. The following reactions are fun examples to try. (See the Selected Web Resources on page 3 of this document for detailed instructions.) Turn water into “wine,” “milk,” and “beer” using common chemicals. Mix calcium chloride, baking soda, water, and phenol red in a resealable bag. React copper wire (Cu) with a silver nitrate solution (AgNO3). React a metal such as magnesium (Mg) or zinc (Zn) with hydrochloric acid (HCl). Follow all suggested safety guidelines, and be sure that students wear appropriate safety equipment (goggles, apron/lab coat, latex gloves) when handling chemicals. Individual chemicals and reaction kits can be ordered from science-supply companies. Alternatively, you can use the Internet to show videos of spectacular reactions. This is much safer but definitely not as much fun as the real thing! 2018 2. Prior to using the Gizmo ( 10 – 15 minutes) Before students are at the computers, pass out the Student Exploration sheets and ask students to complete the Prior Knowledge Questions. Discuss student answers as a class, but do not provide correct answers at this point. Afterwards, if possible, use a projector to introduce the Gizmo and demonstrate its basic operations. 3. Gizmo activities ( 15 – 20 minutes per activity) Assign students to computers. Students can work individually or in small groups. Ask students to work through the activities in the Student Exploration using the Gizmo. Alternatively, you can use a projector and do the Exploration as a teacher-led activity. 4. Discussion questions ( 15 – 30 minutes) As students are working or just after they are done, discuss the following questions: How does making s’mores relate to balancing chemical reactions? Why do chemical equations have to be balanced? Why are you allowed to change the coefficients but not the subscripts? What are some real-world examples of chemical reactions that you can think of? [Possible examples may include burning gas or wood, rusting iron, tarnishing silverware, photosynthesis, and reaction of baking powder in cake batter.] 5. Follow-up activity: Classifying reactions ( 45 – 60 minutes) Give examples of real chemical reactions, and ask your students to balance and classify the chemical equations. Here are five examples you can demonstrate in class: Synthesis: ___ Mg + ___ O2 ___ MgO Decomposition: ___ C12H22O11 ___ C + ___ H2O Single replacement: ___ Zn + ___ HCl ___ ZnCl2 + ___ H2 Double replacement: ___ Pb(NO3)2 + ___ NaI ___ NaNO3 + ___ PbI2 Combustion: ___ C4H10 + ___ O2 ___ CO2 + ___ H2O The “Reaction types demo” link in the Selected Web Resources describes how to present these reactions to your class. Please read and follow all safety instructions carefully. The decomposition reaction should be performed in a laboratory hood. If you do not have access to a hood, you can try the “Old Foamey” demonstration, which shows the decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2). Scientific Background A chemical reaction occurs when substances, called reactants, are mixed and changed to a new set of substances, or products. In this process, chemical bonds between atoms are broken and new bonds are formed, but the atoms themselves remain intact. The law of conservation of matter states that in a chemical reaction, no matter is created or destroyed. (Note: In a nuclear reaction, some matter is converted to energy.) In most cases, a certain amount of energy, called activation energy, is required to get the reaction started. The reaction often releases energy as well. If the activation energy is greater 2018 than the released energy, the reaction is endothermic. An exothermic reaction releases more energy than is required to start the reaction. Chemical reactions can be classified into five categories. In a synthesis reaction (also called a combination reaction), two or more substances combine to form a single substance. In a decomposition reaction, a single substance breaks down to form two or more substances. In a single replacement reaction, a compound reacts with an element to form a new compound and a different element. In a double replacement reaction, two compounds exchange parts. Combustion reactions involve burning a fuel (often an organic compound) in oxygen, usually producing carbon dioxide and water. Balancing chemical reactions is useful because it allows you to calculate how much of each reactant you will need to produce a desired amount of product. Scientists use the mole (Mol) to measure amounts of substances. A mole of a substance has a mass in grams that is equal to the atomic mass of one molecule of the substance. For example, water has an atomic mass of 18 amu (atomic mass units), so a mole of water is 18 grams of water. Based on the balanced equation: 2H2 + O2 2H2O You can deduce that it requires 2 moles of hydrogen gas (4 grams) and 1 mole of oxygen gas (32 grams) to produce 2 moles of water (36 grams). Environmental connection: Energy from hydrogen If an electric current is passed through water, hydrogen and oxygen gas are produced. A fuel cell does the opposite, producing an electric current by combining oxygen and hydrogen gas. Like a battery, a fuel cell is a clean and efficient source of energy; it emits only water. While oxygen is plentiful in Earth’s atmosphere, hydrogen is only found in combination with other substances. The only way to isolate a usable amount of hydrogen gas is to extract hydrogen from these other compounds, which requires energy. Therefore, hydrogen is not a primary source of energy like coal or gas. Instead, it is a way to store and transport energy. Clean methods of producing hydrogen include using energy from windmills or solar panels to extract hydrogen from water. Selected Web Resources Chemical reactions: http://www.chem4kids.com/files/react_intro.html Balancing reactions: http://www.chemteam.info/Equations/Balance-Equation.html Demonstrations and Labs: Reaction types demo: http://www.scienceteacherprogram.org/biology/Cork07.html Flaming bubbles: https://www.bealsscience.com/single-post/2017/10/17/Flaming-SoapBubbles---Holding-a-Fireball-in-my-Hand Calcium chloride/baking soda: http://chemistry.elmhurst.edu/demos/reactioninbag2.htm Mg +HCl: http://chemdemos.uoregon.edu/demos/Magnesium-and-Hydrochloric-Acid Color changes: http://chemistry.about.com/cs/demonstrations/ht/blwwmbdemo.htm Fuel cells: http://hfcarchive.org/fuelcells/ Chemical Equations Gizmo: http://www.explorelearning.com/gizmo/id?461 Chemical Changes Gizmo: http://www.explorelearning.com/gizmo/id?1060 Collision Theory Gizmo: http://www.explorelearning.com/gizmo/id?553 Limiting Reactants Gizmo: http://www.explorelearning.com/gizmo/id?365 Stoichiometry Gizmo: http://www.explorelearning.com/gizmo/id?515 2018

