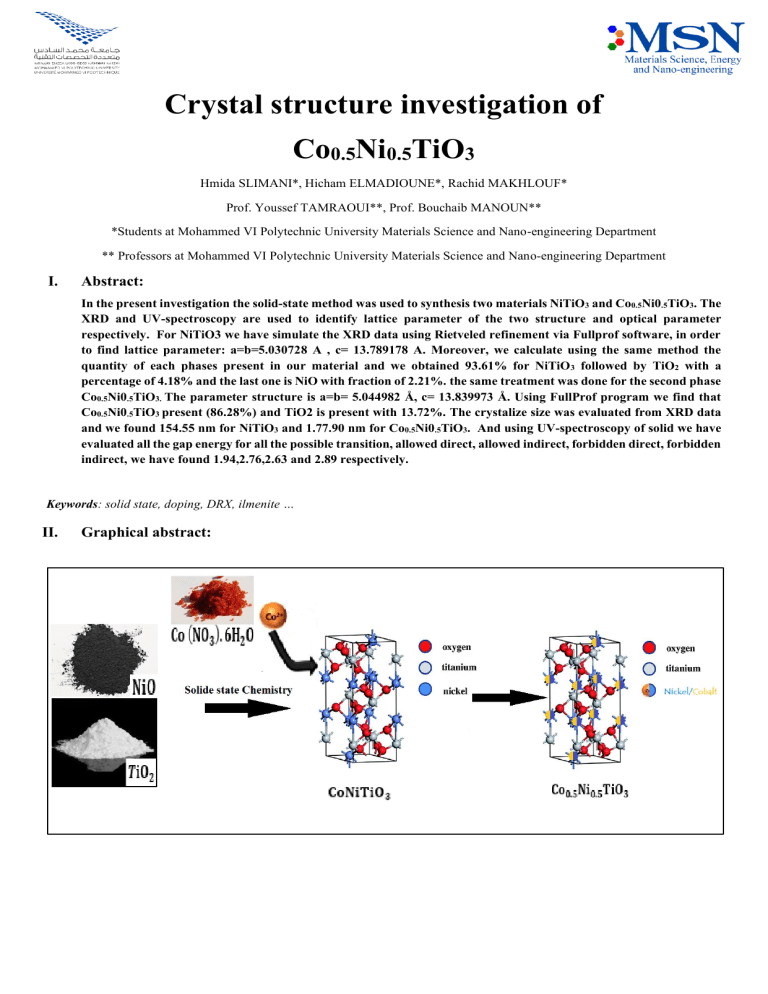
Crystal structure investigation of Co0.5Ni0.5TiO3 Hmida SLIMANI*, Hicham ELMADIOUNE*, Rachid MAKHLOUF* Prof. Youssef TAMRAOUI**, Prof. Bouchaib MANOUN** *Students at Mohammed VI Polytechnic University Materials Science and Nano-engineering Department ** Professors at Mohammed VI Polytechnic University Materials Science and Nano-engineering Department I. Abstract: In the present investigation the solid-state method was used to synthesis two materials NiTiO3 and Co0.5Ni0.5TiO3. The XRD and UV-spectroscopy are used to identify lattice parameter of the two structure and optical parameter respectively. For NiTiO3 we have simulate the XRD data using Rietveled refinement via Fullprof software, in order to find lattice parameter: a=b=5.030728 A , c= 13.789178 A. Moreover, we calculate using the same method the quantity of each phases present in our material and we obtained 93.61% for NiTiO 3 followed by TiO2 with a percentage of 4.18% and the last one is NiO with fraction of 2.21%. the same treatment was done for the second phase Co0.5Ni0.5TiO3. The parameter structure is a=b= 5.044982 Å, c= 13.839973 Å. Using FullProf program we find that Co0.5Ni0.5TiO3 present (86.28%) and TiO2 is present with 13.72%. The crystalize size was evaluated from XRD data and we found 154.55 nm for NiTiO3 and 1.77.90 nm for Co0.5Ni0.5TiO3. And using UV-spectroscopy of solid we have evaluated all the gap energy for all the possible transition, allowed direct, allowed indirect, forbidden direct, forbidden indirect, we have found 1.94,2.76,2.63 and 2.89 respectively. Keywords: solid state, doping, DRX, ilmenite … II. Graphical abstract: III. Introduction: New structure can come from existing materials, we need just to put the right proportion and use the right method to get them. One method is the solid-state method, the oldest and most used method to produce multicomponent solid material, a lot of structure can be producing from completely different initial structure. Perovskites and ilmenite structures with general formula ABO3 (A=Pb, Ni, Fe, Co….) and (B=Ti,…) can produced using initial reagent with different structure , in the perovskites structures a variation of the ionic radius of B cation (rb) is permitted only to an extent which is defined as the tolerance factor t, perovskites structure forms when t lies between 0.8 and 1, for t value smaller than 0.8 the ilmenite structure is formed . Pressure disordering in the ilmenite structure may cause its breakdown to slightly more dense corundum structure , the perovskites and corundum phases are seen to have higher entropies than the ilmenite , ilmenite adopted a rhombohedral structure (R-3 space group) which is stable at low temperature ,Nickel titanite (NiTiO3) member of ilmenite family have attached attracted great interest over the past few decades because of the utilization of such materials in wide range of application such as photocatalysis , sensor, fuel cell, [1-5] , in NiTiO3 both Ni and Ti atoms prefer octahedral coordination with alternating cation layers occupied by Ni and Ti alone [6] The literature about the properties study of ilmenite materials influencing Co0.5Ni0.5TiO3 materials as a solid solution has rarely been reported. In recent years, NiTiO3, a widely known type of Ti-based ilmenite (MTiO3), has become a key research topic in basic physics and potential technology applications. Nickel titanate is a trigonal crystal system with an ilmenite structure. Ti and Ni atoms are spaced in the cationic layer of octahedral coordination. NiTiO3 is a bright candidate with a narrow band gap; it is an n-type semiconductor with a band gap of approximately 2.18 eV [7,8]. In this manuscript, we report the synthesis, the optical propriety, and Reitveld refinement of CoxNi1-xTiO3 by solid state chemistry. IV. Materials and methods a. Synthesis procedure / protocol The synthesis of oxides by solid state reaction is one of the most used methods in solid state chemistry and in industries, thanks to its simplicity and its large-scale production. We have used this method to elaborate our perovskite compound Co0.5Ni0.5TiO3 following steps: • Reagents Table 1:Reagents used in our study Reagent Formula M (g/mol) Titanium oxide TiO2 79,86 Nickel oxide NiO 74,69 Cobalt nitrate hexahydrate Co (NO3).6H2O 291,04 The reagents in the form of powders (Table1), are weighed in stoichiometric quantities predicted by the reaction. • Reaction 𝑻𝐢𝐎𝟐 + 𝐍𝐢𝐎 + 𝐂𝐨 (𝐍𝐎𝟑 ). 𝟔𝐇𝟐 𝐎 → 𝐂𝐨𝟎.𝟓 𝐍𝐢𝟎.𝟓 𝐓𝐢𝐎𝟑 + 𝐇𝟐 𝐎 Then we mixed them carefully by grinding in a mortar. The obtaining homogeneous mixture composed of small particles will then facilitate the kinetics of the reaction, the mass obtained is 1.815 g. The powder is then subjected to calcination which is a successive heat treatment, firstly the temperature retained is 450°C for 1h, then temperature is raised to 650°C for 4h and by solid phase diffusion phenomena the materials will react until a single phase is obtained, the final mass obtained is 1,760 g. So, the degradation mass is: md = 1.815 – 1.760 = 0.055 g Since it consists of some impurities, we have been reground in order to reduce the size of the grains and increase its reactivity. The powder is then subjected again to a heat treatment at high temperature which is 850°C for around 5h, then it increases to 1000 °C in order to finally obtain the desired product. b. Characterization techniques (XRD) X-ray diffraction (XRD) is a technique for characterizing crystallized materials, whether massive, in the form of powder or deposits. In the laboratory, this technique is mainly applied to inorganic materials: minerals, metals, alloys, ceramics, etc. The principle is as follows: The X-ray beams produced by the tube are sent to the sample in which they are deflected by the atoms. These diffracted beams interfere with each other, leading to the production of an intense signal in certain precise areas of space. It is this signal which is collected by the detector and plotted in the form of a curve (diffractogram) which presents peaks at very specific angles of diffraction. The position of these peaks is a real signature of the arrangement of atoms inside a crystal (distance between atoms, between intracrystalline planes). The empirical relationship between the angles at which peaks are observed and the distances between atomic planes is Bragg's law. nλ=2d sin θ c. Rietveld Method The aim of Rietveld refinement is to find a set of powder diffraction simulation parameters which lead to optimum agreement between a simulated powder diffraction pattern and experiment. The refined parameters provide information about the crystal structure and other properties of the powder sample and instrument setup. The agreement between simulation and experiment is quantified by a measure of similarity. Rietveld refinement are also used to refine parameters affecting peak shapes and positions, such as profile parameters, sample broadening, asymmetry parameters, line shift corrections, cell parameters, and so on The Rietveld program implemented in the Powder Refinement tool is, for the most part, based on standard algorithms employed in Rietveld refinement. Robustness is achieved by ensuring that refineable parameters remain within physically sensible ranges. It is generally difficult to generalize refinement strategies since every refinement has its own problems. Nevertheless, it is useful for the novice to sequence the refineable parameters. A typical refinement based on a single-phase constant wavelength X-ray data set might proceed as follows: 1. 2. 3. 4. 5. 6. 7. 8. Scale and background Lattice parameters Peak shape Fractional coordinates Isotropic thermal parameters Fractional occupancies Anisotropic thermal parameters Preferred orientation extinction and absorption parameters Before starting the refinement, we need several information, unit cell dimension, Z, space group, atomic coordinates, X-ray wavelength, approximate peak shape parameters (pseudo-Voigt for XRD) Therefore, a structural model has to be input into the refinement in order to calculate structure factors, the starting model may be found in a number of ways, a known structure or isostructural analogue, calculated by ab-initio techniques. There are several Rietveld refinements packages, in our study we use the FullProf package. Refinement is normally continued until and acceptable fit is obtained visually, or some convergence criteria have been pattern….) V. found (R-pattern, Results and data treatments 1. NiTiO3 structure: a. Overview about XRD Patterns NiTiO3 B 30000 Intensity (a.u) 25000 20000 15000 10000 5000 0 20 30 40 50 60 70 2 Theta(°) Figure 1: XRD spectra of NiTiO3 80 90 100 R-weighted To have a general idea about this structure we have use cycles of refinement, we got Bragg R-factor: 12.54 and Highscore. Comparing our XRD with those of literature RF-factor: 11.52 and steel some pics of the other phase we found that we have extra peaks. It means that we to fit. Then we added the second abundant phase TiO2 have other phases. which we fitted using its structural parameters taken from HighScore program. The refinement in this time Table 2:Peaks of other phases present in our phase reduce the the Bragg R-factor: 7.8 and RF-factor: 8.22 2 Theta Phase 27.63 TiO2 36.28 37.41 54.33 TiO2 NiO TiO2 of the first phase. However, the second’s phase factors were inacceptable, the Bragg R-factor: 21.15 and RFfactor: 25.04. we have tried to change the model of TiO2 many times, but it was the best result we got. Finally, we decide to add the last phase NiO, the same with TiO2 Frome the table below we believe that we have the we extract its structural parameters from HighScore. formation of side phase TiO2 and we steel have amount The addition of this phase, all missing pics were of NiO not reacted during the solid-state reaction. We included within the refinement process. And the the have obtained thin peaks in our XRD structure which Bragg R-factor and RF-factor were dramatically mean our product has a high crystallinity. Figure 1 show reduced to: the XRD spectra of NiTiO3. b. ➢ Phase: Crystal structure analysis (Rietveld refinement): 1 ✓ Bragg R-factor: 2.121 The first thing we have done is much our structure with ✓ RF-factor: 2.232 other structure in literature using the high score ➢ Phase: program. We found that we have 3 phases NiTiO3 which 2 ✓ Bragg R-factor: 3.393 present 93.61% of the structure and TiO2 with a ✓ RF-factor: 4.606 percentage of 4.18% and the last one is NiO with ➢ Phase: fraction of 2.21%. 3 ✓ Bragg R-factor: 3.462 We start refining the abundant phase NiTiO3 which we ✓ RF-factor: 1.963 used its CIF file to facilitate the the task. After many NiTiO3 Yobs Ycalc Yobs-Ycalc Bragg_position 30000 25000 20000 Yobs 15000 10000 5000 0 -5000 -10000 20 30 40 50 60 70 80 90 100 110 ! 2 Theta (deg.) Figure 2: Plot of Rietveld refinement of NiTiO3. The upper symbols illustrate the observed data (red circles) and the calculated pattern (solid line). The vertical markers show calculated positions of Bragg reflections. The lower curve is the difference diagram c. Rietveld refinement conditions Table 3:Details of Rietveld refinement conditions of the series NiTiO3 Composition Symmetry Space group z Lattice parameters Volume P-V function Caglioti parameters RF/RB RP/Rwp cRP/cRwp d. Phase1:NiTiO3 Phase2: TiO2 Phase 3: NiO Hexagonal R -3 1 a=b= 5.030728 c= 13.789178 302.225 A3 0.50000 U= 0.007790 V= -0.001655 W= 0.002201 Tetragonal P 42/m n m Cubic F m -3 m a=b= 4.593339 c= 2.959635 62.445 A3 0.50000 U= 0.019805 V= -0.007899 W= 0.003344 a=b=c= 4.177288 1.89/2.12 4.93/ 6.48 10.9/ 10.5 4.61/ 3.39 ------- 1.96/ 3.46 ------- 72.893 A3 0.50000 U= -0.009983 V= 0.011890 W= 0.000430 Structural parameters Table 4: Refined structural parameters for NiTiO3 with R -3 as space group. Ti Ni O Site NiTiO3 x y z Biso Occupancy 6c 0 0 0.14463 0.50000 1.00000 6c 0 0 0.35075 0.50000 1.00000 18f 0.31643 0.01831 0.24522 0.50000 3.00000 2. Co0.5Ni0.5TiO3 structure: Table 5: Selected inter-atomic distances (Å) in the series NiTiO3 0.0 Composition 6c site with Coordination number = 6 2.0784 Ti—O1 1.8523 Ti—O2 1.9654 <Ti—O> 2.1245 Ni—O1 2.0585 Ni—O2 2.0915 < Ni—O1> 18f site with coordination number 4 2.0784 O1-- Ti 1.8523 O1-- Ti 2.1245 O1-- Ni 2.0585 O1-- Ni 2.0284 Average dis Tables 4 and 5 represent the refined structural parameters and inter atomic distance in the series respectively for NiTiO3. We can see clearly that the distance between O and Ni is greater than the distance between O and Ti. a. Overview about XRD Patterns The same thing we have done for the first structure. We will use fullprof software to define the phases present in our product Table 6:Peaks of other phases present in our phase 2 Theta Phase 27.57 TiO2 36.20 TiO2 41.26 TiO2 54.35 TiO2 56.65 TiO2 In this case we see that the only phase side phase obtained is TiO2. And we don’t have NiO in the final structure we can assume that it reacts totally in this case because we have used half amount this time. Like the first structure we have obtained thin peaks. Therefore, our structure has a high crystallinity. Figure 3 show the XRD spectra of the structure. Co0.5Ni0.5TiO3 16000 14000 Intensity (a.u) 12000 10000 8000 6000 4000 2000 0 -2000 20 30 40 50 60 70 80 90 100 2 Theta (°) Figure 3:XRD spectra of Co0.5Ni0.5TiO3 b. Crystal structure analysis (Rietveld refinement): Co0.5Ni0.5TiO3 20000 Yobs Ycalc Yobs-Ycalc Bragg_position Yobs 10000 0 10 20 30 40 50 60 70 80 90 100 110 ! 2 Theta (deg.) Figure 4:Plot of Rietveld refinement of Co0.5Ni0.5TiO3. The upper symbols illustrate the observed data (red circles) and the calculated pattern (solid line). The vertical markers show calculated positions of Bragg reflections. The lower curve is the difference diagram The main idea was to use the PCR file generated in the undoped structure NiTiO3, but the model didn’t work much and were not able to refine using this PCR file. Therefor we start the refinement from the beginning this time. The first thing we have done is analysing our structure using High score software, in order to define how many phases, present in this product. We found that we have in parallel with NiTiO3 doped phase with 0.5 of Co we have another phase which correspond to TiO2 phase. This explain why the PCR file of NiTiO3 does not work, in this product we do not have NiO phase which could deform the refinement process. Using FullProf program we refined firstly the abundant phase Co0.5Ni0.5TiO3 (86.28%). And after many cycles of refinements, we got a Bragg R-factor: 10.5 and RFfactor: 11.33 That we could not make it better because some pics were not fitted (Figure 4). Then we add the second phase TiO2 (13.72%) using structural parameters obtained in HighScore. Finally, all pics were included and well fitted in the refinement and we have reduced the: Phase1: ➢ Bragg R-factor: 1.941 ➢ RF-factor: 3.517 Phase 2: ➢ Bragg R-factor to: 3.066 ➢ RF-factor to: 3.659 c. Rietveld refinement conditions Table 7:Details of Rietveld refinement conditions of the series Co0.5Ni0.5TiO3 Composition Symmetry Space group z Lattice parameters Phase 1: Co0.5Ni0.5TiO3 Hexagonal R -3 1 a=b= 5.044982 c= 13.839973 305.060 A3 0.12379 U= 0.010088 V= -0.001821 W= 0.001887 2.94/ 1.92 5.77/ 7.52 14.2/ 13.1 Volume P-V function Caglioti parameters d. RF/RB RP/Rwp cRP/cRwp Structural parameters Phase 2: TiO2 Tetragonal P 42/m n m a=b= 4.593287 c= 2.959840 62.448 A3 0.12379 U= 0.000818 V= 0.001668 W= 0.001951 3.66/ 3.07 ------- Table 8:Refined structural parameters for NiTiO3 with R -3 as space group. Ti Ni Co O Site NiTiO3 6c 6c 6c 18f x y z Biso Occupancy 0 0 0 0.31750 0 0 0 0.02060 0.14496 0.35200 0.35200 0.24490 1.15755 1.63431 1.63431 1.86482 1.00000 1.00000 1.00000 3.00000 Table 9:Selected inter-atomic distances (Å) in the series Co0.5Ni0.5TiO3 0.0 Composition 6c site with Coordination number = 6 2.0792 (32) Ti—O1 2.0792 (39) Ti—O1 2.0792 (29) Ti—O1 1.8507 (25) Ti—O2 1.8507 (34) Ti—O2 1.8507 (40) Ti—O2 1.9650 <Ti—O> 2.1464 (32) Ni—O1 2.1464 (38) Ni—O1 2.1464 (29) Ni—O1 2.0663 (40) Ni—O2 2.0663 (26) Ni—O2 2.0663 (32) Ni—O2 2.1064 < Ni—O1> 2.1464 (32) Co—O1 2.1464 (38) Co—O1 2.1464 (29) Co—O1 2.0663 (40) Co —O2 2.0663 (26) Co —O2 2.0663 (32) Co —O2 2.1064 < Co —O1> 18f site with coordination number 6 2.0792 (32) O1-- Ti 1.8507 (25) O1-- Ti 2.1464 (32) O1-- Ni 2.0663 (40) O1-- Ni 2.1464 (32) O1—Co 2.0663 (40) O1-- Co 2.0592 Average dis e. Crystallite size calculation: The crystallite size was evaluated form XRD Data using Scherer’s Formula: 𝐷𝑝 = 𝐾𝜆 𝐵𝑐𝑜𝑠(𝜃) We have Select 4 pics for each compound we summarize the result in the following table: Compound Peak position 2θ (°) FWHM size (°) NiTiO3 Ni0.5Co0.5TiO3 f. 33.134361 0.064423 35.68793 0.055439 54.023376 0.06014 24.169256 0.049413 33.024391 0.055445 35.586544 0.04357 24.097744 0.047754 24.09758 0.047702 B Dp Average (nm) 154.55 177.90 Structure view: Where, R is the diffuse reflectance and F(R) is called the Kubelka-Munk function which is proportional to absorption coefficients ‘A’. The optical band gap energy was then estimated using the onset of the absorption edge by Tauc plots [11]. The predominant mechanism of the band to band transitions, which are responsible for the shape of the absorption edge, was determined by investigation of the dependence F(R) as a function of the incident photon energy (hν). The relation between the absorption coefficient and hν can be determined using Tauc's relationship in the high absorption region [11,12]: F ( R)h = A(h − Eg )n Where, F(R) is the linear absorption coefficient or the Kubelkamunk function, υ= C/λ is the light frequency, with λ being the wavelength (meter) and C is the speed of light =3.0×108 (meter/sec). h = Planks constant = 6.626×10-34 Joules sec, A is a constant depending on the transmittance properties of Eg. The index n is determined by the nature of the electron transition during the absorption process. This index determines the transition nature; it’s also called power of probabilities. Four possible values which are 1/2, 2, 3/2 or 3 corresponding respectively to direct allowed, indirect allowed, direct forbidden and indirect forbidden types. In order to determine optical band gap (Eg) experimentally, [F(R)hν] n (n=1/2, 2, 3/2 or 3) versus the photon energy (hν) can be plotted using the data obtained from the optical absorption spectra. As a result, and according to Tauc plot the straight line was extrapolated to intercept (hν)-axis at [F(R)hν] ½ = 0 VI. Optical measurement (band gap calculation): Band gap Eg is a tunable property that is affected by numerous parameters such as synthesis root and thermodynamic conditions, surface morphology, crystal structure, atomic substitution Ratio, and the degree of distortion.The theory of Kubelka-Munk function was proposed by P. Kubelka and F. Munk [9,10] to calculate the Eg from the reflectance spectra, according to: (1 − 𝑅)2 𝐹(𝑅) = 2𝑅 %R 0.3 0.2 0.1 200 300 400 500 600 700 wave length (nm) Figure 5: Co0.5Ni0.5TiO3 UV/Vis Spectrum obtained in Reflectance mode 800 Figure 8:(αhν)n (n=1/2, 2, 3/2 or 3) versus (hν) for the prepared material. Fig.7 show the evolution of absorption coefficient (KM function) in the range of wavelength between 250 and 800 nm, where two absorption bands are clearly identified at 400 and 600 nm. 6 K-M function F(R) 5 4 Fig.8 shows the plots of (αhν) prepared material. 3 2 1 0 200 300 400 500 600 700 800 n versus (hν) for the We have determined the bandgap for our compound of all the transition, we Summarize the result in the following table. wave length (nm) Figure 6:UV-vis-near-IR Spectrum of Co0.5Ni0.5TiO3 sample, showing the evolution of absorption coefficient (K-M function) in the range of wavelength between 250 and 800 nm. In Fig.6 we can see the reflectance spectra for our sample, where two absorption bands are clearly identified at 500 and 700 nm. Type of Transition Direct allowed Indirect allowed Direct forbidden Indirect forbidden Gap Energy (eV) 1.94 2.76 2.63 2.89 VII. Conclusion The MTiO3 has received an attention for their use in application such as in electric field. During this work we have applied the solid-state reaction to prepare Nickel titanate and nickel cobalt titanate (Co0.5Ni0.5TiO3). the two structure are treated the same way. To analyse our product, we have used XRD to identify the crystallinity of materials and the phases formed, and UVspectroscopy in order to measure the gap bond of each structure. The XRD analysis show that the NiTiO3 structure contains two other phases TiO2 which was formed during the heat treatment, and NiO which correspond to the amount of reactant does not reacts. Those result was confirmed by HighScore software. The second structure (Co0.5Ni0.5TiO3) involves only one parasitic phase TiO2. Both structures give XRD spectra with thin peaks. Therefore, both are highly crystalline. Moreover, we have used Rietveld refinement via Fullprof software in order to find structural parameters of a material, and to quantitatively examine the mixing ratio of each phase in the material. And as we have mentioned earlier those structures are in electric field so it’s necessary to measure their band gap using UV-spectroscopy of solid and we have found 1.94 eV for Co0.5Ni0.5 TiO3 which correspond to semiconductive materials References: [1] I. V. Pishch and E. V Radion, "Synthesis of pigments based on perovskite" Glass and Ceramics. 1998; 55: 290291.. [2] X. Lin, J. Xing, W. Wang, Z. Shan, F. Xu, F. Huang, "Photocatalytic Activities of Heterojunction Semiconductors Bi2O3/BaTiO3: A Strategy for the Design of Efficient Combined Photocatalysts" J. Phys. Chem. C 2007; 111: 18288–93. [3] Y. Lin, Y. Chang, W. Yang, B. Tsai, "Synthesis and characterization of ilmenite NiTiO3 and CoTiO3 prepared by a modified Pechini method" J Non-Cryst. Solids 2006; 352: 789-4. [4] A. Montenero, M. Canali, G. Gnappi, D Bersani, P.P. Lottici, P. Nunziante, E.Traversa, "Structural and Electrical Properties of Sol–Gelprocessed CdTiO3 Powders and Films" Appl. Organomet. Chem. 1997; 11: 137-146. [5] X. Zhang, H. Wang, A. Huang, H. Xu, Y. Zhang, D. Yu, B. Wang, H. Yan "Synthesis of cadmium titanate powders by a sol-gel-hydrothermal method" J. Mater. Sci. 2003; 38: 2353-2356. [6] N. Yonghong, W. Xinghong, H. Jianming, "Nickel titanium microtubes constracted by nearly spherical nanoparticales preparation, characterization and properties" Materials research Bulltin2009; 44: 1787 – 1803. [7] M.A. Ruiz-Preciado, A. Kassiba, A. Gibaud, A. MoralesAcevedo, Comparison of nickel titanate (NiTiO3) powders synthesized by sol-gel and solid-state reaction, Mater. Sci. Semicond. Process. 37 (2015) 171–178. [8] T.T. Pham, S.G. Kang, E.W. Shin, Optical and structural properties of Mo-doped NiTiO3 materials synthesized via modified Pechini methods, Appl. Surf. Sci. 411 (2017) 18– 26. [9] A. Escobedo Morales, E. Sánchez Mora, U. Pal, Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures, Rev. Mex. Fis. Suppl. Vol. 53, No. 5, p.18-22. 53 (2007) 18– 22. [10] P. Kubelka, F. Munk, An Article on Optics of Paint Layers (engl. Übersetzung), Z. Tech. Phys. 12 (1931) 593–601. [11] J. Tauc, Optical properties and electronic structure of amorphous Ge and Si, Mater. Res. Bull. 3 (1968) 37–46. [12] G.P. Joshi, N.S. Saxena, R. Mangal, A. Mishra, T.P. Sharma, Band gap determination of Ni-Zn ferrites, Bull. Mater. Sci. 26 (2003) 387–389.






