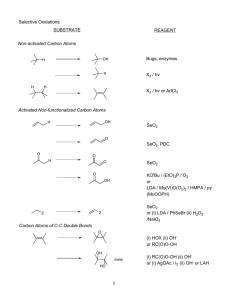
CHAPTER-3 PERICYCLIC REACTIONS T he Pericyclic Reactions involve a cyclic redistribution of bonding electrons through a concerted process i.e. without intermediates. It is concerted processes that proceed via a cyclic transition state. 3.1. Classification of Pericyclic Reactions 1. Electrocyclic Reactions: Electrocyclic Reactions are reversible by nature, and usually involve ring opening or ring closing. Electrocyclic Reactions could also be referred as concerted cyclization of a conjugated π-electron system by converting one π-bond to a ring forming σ-bond. It is an intramolecular reaction in which a new σ (sigma) bond is formed between the ends of a conjugated π system. For e.g. TRICK 101: Product is a cyclic compound that has one more ring and one less π bond than the reactant. In the reverse direction, σ bond in cyclic reactant breaks forming conjugated π system. It has one more π bond than the cyclic reactant. For e.g. 2. Cycloaddition Reaction: In this reaction, two different π-bond containing molecules react to form cyclic compound. It is designated as [A + B] where A and B refers to the number of atoms containing π - electrons. Each of the reactant loses a π – bond and resulting cyclic product has two new σ – bonds. For e.g., TRICK 101: The number of participating π-electrons in each component is given in brackets preceding the name 3. Sigmatropic Reaction: In this reaction, a σ bond is broken in the reactant and a new σ bond is formed in the product and the π – bond does not change but rearrange. σ bond that is broken can be in the middle of π system or at the end of π – system. TRICK 101: Electrocyclic and sigmatropic rearrangement are intramolecular reactions while cyclo-addition is an intermolecular reaction. 3.2. Molecular Orbital Theory 1. Conservation of Orbital Symmetry Theory: It states that in – phase orbitals overlaps during a pericyclic reaction. It was based on frontier orbital theory which is based on molecular orbitals and orbital symmetry. 2. Frontier Orbital Method: Analysis is based on the interaction of HOMO of one component with LUMO of other component. HOMO – highest occupied molecular orbital – filled LUMO – lowest unoccupied molecular orbital – empty a. Molecular Orbital Description of Ethene: It has one π bond, it has two p atomic orbitals that combine to form two π molecular orbital. The two p-orbitals in similar phase overlap generating a π bonding molecular orbital, denotes as ψ1, it is occupied with two low energy bonding molecular orbital. Π* antibonding molecular orbital is formed as two p-orbitals in opposite phase combine generating a destabilizing node b. Molecular Orbital Description of 1,3-Butadiene: It has two conjugated π – bond so it has four p – atomic orbitals. These four different p-orbitals combine in different manner to generate four different molecular orbital ranging from ϕ1- ϕ4. Two of these are bonding molecular orbital namely ϕ1 and ϕ2, and lower in energy than the parent p-orbital. There are two antibonding orbital namely ϕ3 and ϕ4 and these are higher in energy than the parent p-orbital. where, S = Symmetric Molecular Orbital, A = Asymmetric Molecular Orbitals (i) Symmetric Molecular Orbitals: If the p – orbitals at the end of molecular orbital have same sign (in-phase) then symmetric. For e.g. ψ1 in 1, 3 – Butadiene has same (+) sign of upper lobe of first and fourth p – orbital and (–) sign of lower lobe (ii) Asymmetric Molecular Orbital: If the p – orbitals at the end of molecular orbital have opposite sign (out of phase). For e.g. - ψ2 has (+) and (–) sign respectively of upper lobe of first and fourth p – orbital and hence asymmetric. TRICK 101: Ground state HOMO and excited state HOMO always have opposite symmetries; one is symmetric then other is asymmetric. c. Molecular Orbital Description of 1, 3, 5 – hexatriene: The structure of hexatriene is as follows: Six p-orbitals combine differently to form six molecular orbitals denoted as ψ1- ψ6. Amongst these three are bonding molecular orbital (ψ1- ψ3) and the other three are antibonding molecular orbital (ψ4- ψ6). However, it must be noted that, 1. For the ground state electronic configuration, six π-electrons occupy three bonding orbitals, hence, ψ3 is the HOMO and ψ4 is the LUMO. 2. For excited state, electrons from ψ3 go to ψ4, thus, ψ4 is the HOMO and ψ5 is the LUMO 3.3. Electrocyclic Ring Closure/Ring Opening The following can happen in two manners. They are noted below: 1. Conrotatory Closure: In this case, the terminal p – orbitals rotate in the same direction. The like phases of p-orbitals lie on the opposite side of the molecule, the two orbitals must orient themselves in the same direction clockwise – or anticlockwise; For instance, TRICK 101: If HOMO is asymmetric (end orbitals are different) then rotation has to be conrotatory in order to achieve in – phase overlap on say bonding situation. 2. Disrotatory Closure: In this case, the terminal p – lobes rotate in opposite direction. The like phases of p-orbitals lie on the same side of the molecule, the two orbitals must orient themselves in the opposite direction one clockwise, another anticlockwise. For instance, TRICK 101: If the HOMO is symmetric (the end orbitals are identical), rotation will be disrotatory in order to achieve in – phase overlap i.e. bonding situation. IMPORTANT: Case 1: If HOMO is symmetric Then Disrotatory ring closure is symmetry allowed, whereas conrotatory ring closure is symmetry forbidden. Case 2: If HOMO is asymmetric Then Disrotatory ring closure is symmetry forbidden while conrotatory ring closure is symmetry allowed. 3. a. Stereochemistry of Product and Mode of rotation: Consider the following case: ❖ For (2E, 4Z, 6E) – octatriene: It has three π – bonds, so 6 p – orbitals and HOMO is ψ3 in the ground state which is symmetric, thus disrotatory ring closure is allowed. 3.3.1. Photochemical & Thermal Reactions 1. Photochemical Reactions: It takes place when a reactant absorbs light. It involves excited state molecular orbitals (HOMO and LUMO). 2. Thermal Reactions: It takes place at room temperature and ground state molecular orbital (HOMO and LUMO) are involved. ❖ What is the product of Ring Closure of (2E, 4Z, 6Z) – octatriene if reaction takes place under thermal conditions? Ans: In Thermal condition, ground state HOMO ψ3 is involved which is symmetric, so it is disrotatory ring closure. ❖ What is nature of product when the ring closure of (2E, 4Z, 6Z) – octatriene takes place under photochemical condition? Ans: In photochemical condition, excited state HOMO is involved which is ψ4 and it is asymmetric, so conrotatory mode of ring closure is allowed. For (2E, 4Z) – hexadiene: It is similar to 1, 3 – Butadiene but here it is methyl substituted. So its ground state HOMO ψ2 is asymmetric so ring closure is by conrotatory mode under Thermal condition. ❖ What is the product when (2E, 4E) – hexadiene undergo thermal reaction? Ans: The product has been illustrated below: 3.3.2. Woodward-Hoffmann Rules for Electrocyclic Reactions Number of Conjugated π- Reaction Conditions bonds Even Number Odd Number Allowed Mode of Ring Closure Thermal Conrotatory Photochemical Disrotatory Thermal Disrotatory Photochemical Conrotatory 3.6. Configuration of the product in an Electrocyclic Reaction Substituent in the Reactant Mode of Ring Closure Allowed Mode of Ring Closure Point in the Opposite Disrotatory Cis Direction Conrotatory Trans Point in the Same Direction Disrotatory Trans Conrotatory Cis 3.4. Cycloaddition Reaction It is the reaction of two components to form a cyclic compound or a new ring. It is designated as [A + B] where A & B refers to number of atoms containing π – electrons. They can be intermolecular or intramolecular. SAMPLE 3.4.1. Classification of Cycloaddition Reaction 3.4.3. Diels Alder Reaction It is a reaction between a conjugated diene and dienophile. It is a concerted [4 + 2] Cycloaddition reaction. Both enthalpy and entropy decreases. Many Diels – Alder reactions are accelerated by Lewis acid catalyst. For Diels Alder reaction to occur proper alignment of frontiers orbital of diene and dienophile is necessary. The thumb rule should be the positive end of one reactant lines up with negative end of the other reactant. 3.4.4. Classification of Diels Alder Reaction 1. Normal [4 + 2]: Diene is electron rich and Dienophile is electron poor. 2. Inverse electron – demand [4 + 2]: In this case, Diene is electron poor and dienophile is electron rich. 3. Hetero [4 +2]: In it heteroatom can be a part of diene or dienophile or both. 3.4.5. Criteria for Diene in a Diels Alder Reaction 1. Basic Requirements to be a Diene: (i) The diene could be cyclic or open chain. (ii) The diene should be electron-rich and reactivity should be enhanced by electron donating group substituent. 2. Order of Reactivity of Diene towards maleic anhydride (Dienophile): 3. Conformation of Dienes: Open chair diene can acquire two conformations. For instance, The diene must adopt an s – cis conformation to be reactive. Cyclic diene which adopts s – cis conformation are very reactive. For e.g., TRICK 101: Cyclic dienes which are permanently in s – Trans conformation are unreactive in Diels – Alder Reaction. For e.g., 3.4.6. Criteria for Dienophile in Diels Alder Reaction 1. Basic Requirements to be a Dienophile: (i) Please note that a dienophile must not always require to be an alkene, the sole criteria should be containing a π-bond. It could very well be an alkyne. Infact N=N could also participate in the process as long as it carries an electron poor group. (ii) Dienophile should be electron deficient and reactivity enhanced by electron withdrawing substituents. For e.g., 2. Order of Reactivity of Dienophile: a. With Respect to Electron-Withdrawing Substituents: Electron withdrawing substituent increases the rate of reactions. For e.g., b. With Respect to Angle-Strain: The cyclic alkenes and alkynes with angle strain are reactive dienophile. The driving force here is the reduction in angle strain. For e.g., 3.4.7. Stereochemistry of Dienes and Dienophiles SAMPLE 1. Stereochemistry of Dienes: (i) Inside substituents of diene will become β – configuration (above the plane) in the product. (ii) Outside substituents of diene will become α – configuration in the product (below the plane). 2. Stereochemistry of Dienophile: Endo Approach of dienophile is preferred over Exo approach due to secondary orbital overlap between dienophile activating substituent and diene. 3. In a condition where both dienes and dienophiles are substituted, diastereomers are formed which are usually referred as “exo” or “endo” 3.4.8. Regiochemistry of Diels Alder Reaction Please note that when non-symmetrical dienes react with non-symmetrical dienophiles, there occurs a probability of two regioisomers. Dienes with substituents on the terminus (“1-substituted dienes”) tend to give “1,2” products (“ortho”). Dienes with substituents on the 2-position (“2-substituted dienes”) tend to give the “1,4” product (“para”). In general, “1,3” products (“meta”) are only minor byproducts. a. Substituted diene react to give mainly [1, 2] – product: b. Substituted diene react to give mainly [1, 4] – substituted product: ❖ What is the product of following reaction? Indicate with appropriate with appropriate Stereochemistry. Ans: The product would be: The reaction occurs in a following manner: ❖ What is product of following reaction? Ans: The desired product will be: ❖ Predict the structure of the product for the following reaction. Ans: The structure of the product is: ❖ What would be the structure of Product (A) and Product (B)? Ans: ❖ Elucidate the reaction mechanism and structure of the product for the reaction mentioned below Ans: 3.4.9. Intramolecular Diels-Alder Reaction (IMDA) 1. Type I IMDA: Dienophile is attached to C – 1 atom of diene. It results in the formation of fused bicyclic system. It can be elucidated in the following manner: A few examples of Type I IMDA is illustrated below: 2. Type II IMDA: SAMPLE 3.4.10. Hetero Diels-Alder Reaction Hetero-Diels-Alder (HDA) reaction is one of most powerful available methodologies to synthesize optically active six-membered heterocycles, with extensive synthetic applications in natural or unnatural products with a wide range of biological activity. The DA reaction has high regioselectivity and endo-stereoselectivity. In this case, hetero atom is part of the dienophile. For instance, 3.4.11. Retro Diels-Alder or Inverse electron demand D – A Reaction The Diels Alder Reaction is usually reversible in nature. The equilibrium lies by usually toward the Diels-Alder adduct at lower temperature and, at higher temperature, toward the diene and the dienophile. For Retro Diels-Alder reaction, the diene is electron – deficient and the dienophile is electron – rich. High temperatures are normally required for Retro Diels Alder Reaction. ❖ What would be the structure of the product for the reaction mentioned below? Ans: ❖ What would be the structure of the product for the reaction given below? Ans: ❖ Elucidate the structure of the product for the reaction mentioned below. Ans: 3.4.12. Photoenolization When the reactive carbonyl function and a γ-hydrogen are conjugated via an aromatic ring or double bond, the 1,4-diradical created by hydrogen abstraction quickly relaxes to a conjugated enol tautomer. 3.4.13. [2+2] Cycloaddition When compared to [4+2] Cycloaddition Reaction, the [2+2] cycloaddition reaction does not occur under thermal condition (except under revised circumstances), but takes place photochemically. This has been further explained in the sections exploiting the symmetry of HOMO and LUMO of alkene reactant. For [2+2] Cycloaddition to occur the following rules should be taken into account: [2s + 2s] addition not allowed Where s = Suprafacial bond formation [2s + 2s] addition allowed 3.4.15. Stereospecificity of [2+2] Cycloaddition Please note that, Least hindered transition state is observed. 3.4.16. Regioselectivity of [2+2] Cycloaddition 3.4.18. Photochemical [2+2] Cycloaddition Reaction SAMPLE For Photochemical [2+2] Cycloaddition to occur, the light must excite an electron from the ground state HOMO to generate an excited state HOMO which inturn interacts with the LUMO of second alkene to allow the overlap of like phases of p-orbitals. The interaction occurs via suprafacial pathway. 3.5. Sigmatropic Rearrangement 1. Suprafacial Rearrangement: If the migrating group remains on the same face of π – system then rearrangement is Suprafacial. 2. Antarafacial Rearrangement: If the migrating group moves to the opposite face of π system, the rearrangement is Antarafacial. 3.5.1. Classification of Sigmatropic Rearrangements 1. [3,3] Sigmatropic Rearrangement: For [3,3] Sigmatropic Rearrangements, both ends of the relocating σ bond migrate three atoms. These could be broadly divided into two categories, firstly, 'all carbon' version is known as the Cope rearrangement, and an oxygen in the appropriate position changes this to the Claisen rearrangement. [3,3] Sigmatropic Rearrangement can be illustrated through following examples. 2. [1,3] Sigmatropic Rearrangement: [1, 3] Sigmatropic rearrangement involves 2 pair of electrons (one π + one 6 pair of electrons). In it, a sigma bond is broken in the reactant and new 6 – bond is formed and π electrons rearrangement occurs. Thermal [1,3] H-shift reaction cannot take place suprafacially while maintaining bonding interaction between the H and the ends of the π system. Thermal suprafacial [1,3]-sigmatropic H shifts are forbidden. Photochemical [1,3] H-shift reaction can take place suprafacially and maintain simultaneous bonding interactions between the H and the ends of the π system. A photochemical suprafacial [1,3]-sigmatropic rearrangement is allowed (and of course, being suprafacial, is physically possible). TRICK 101: σ – bond that breaks is allylic to carbon. It can be carbon – hydrogen, carboncarbon, carbon – nitrogen bond etc. 3. [2,3] Sigmatropic Rearrangement: For [2,3]- Sigmatropic Rearrangement, five-membered rings in the transition states is observed. This reaction offers great stereoselectivity. The [2,3] Sigmatropic Rearrangement could be illustrated in the following manner: TRICK 101: The E-alkene will favor the formation of anti product, while Z-alkene will favor formation of syn product 4. [1,5] Sigmatropic Rearrangement: For [1,5] Sigmatropic Rearrangement, a six membered transition state ring, with the H atom shifting to a new position is expected . This is a sigmatropic rearrangement since one sigma bond breaks and another forms. The [1,5] hydrogen shift in cyclopentadiene can be observed already at room temperature. At 60°C, migration is so fast that only one signal appears for all hydrogens in 1H-NMR. The [1,5] Sigmatropic Rearrangement could be illustrated in the following manner: IMPORTANT: If the number of pair of electron = even then ground state HOMO is asymmetric. If the number of pair of electron = odd then ground state HOMO is symmetric. 4. [1,7] Sigmatropic Rearrangement: A thermal, concerted [1,7]-H shift is sometimes observed in acyclic systems because the 1,3,5-triene system is floppy enough to allow the H to migrate from the top face to the bottom face (making the triene the antarafacial component). For e.g., 3.5.2. Woodward – Hoffmann Rules for Sigmatropic Reaction Number of Electron-Pairs Reaction Conditions Allowed Mode of Ring Closure Even Number Thermal Antarafacial Photochemical Suprafacial Thermal Suprafacial Photochemical Antarafacial Odd Number 3.5.3. Summary of Thermal Sigmatropic Hydrogen Shift [1,3] H- Shift [1,5] H- Shift [1,7] H- Shift Stereochemistry Antarafacial Suprafacial Antarafacial Feasibility Impossible Easy Possible 3.5.4. [1,3] Sigmatropic Rearrangement The photochemical [1, 3] Suprafacial shift of an alkyl group is allowed with retention of configuration at the migrating carbon. The thermal [1, 3] Suprafacial shift of an alkyl group is allowed with inversion of configuration at migrating carbon and [1 ,3] H shift is forbidden under thermal conditions. [1, 3] – Sigmatropic rearrangements are not common and only seen in system that are highly strained. ❖ What would be the structure of the following product from the reaction given below? Ans: ❖ Elucidate the structure of the product when the following compound is heated. Ans: 3.5.5. Cope Rearrangement It is [3, 3] Sigmatropic rearrangement of a cyclic hexa – 1, 5 – Dienes. A Cope rearrangement is known to take place through a rearrangement of overlap between a group of orbitals around this ring as shown in the example below. Two orbitals forming a sigma bond tilt away from each other while two orbitals that are pi bonding tilt toward each other. It takes place at around 250°C and the activation energy is determined to be 35k cal / mol. 3.5.6. Factors affecting Cope Rearrangement 1. Substituent that can be brought into conjugation during the rearrangement favours it in one direction and lowers the activation energy of such a process. 2. Introduction of alkoxy substitution (oxy – cope system) 3. Release of Strain upon Rearrangement 3.5.7. Stereochemistry of Cope Rearrangement SAMPLE 3.5.11. Stereochemistry of Claisen Rearrangement The Claisen rearrangement is known to be concerted (bond cleavage and recombination), exothermic, pericyclic reaction. The Woodward - Hoffmann rules predict a suprafacial, stereospecific reaction pathway. The kinetics for Claisen Rearrangement are of the first order and it proceeds through a highly ordered cyclic transition state. Crossover experiments tend to eliminate the possibility of the rearrangement which occur through an intermolecular reaction mechanism and are found to be consistent with an intramolecular process. Solvent effects have also been observed in the Claisen rearrangement, the polar solvents tend to accelerate the reaction. Hydrogen-bonding solvents are usually associated with the highest rate constants. For example, ethanol/water solvent mixtures give rate constants 10-fold higher than sulfolane. Trivalent organoaluminium reagents, such as trimethylaluminium, have been shown to accelerate this reaction. Relative Stereochemistry across new carbon – carbon single bond is established as a result of chair like transition state and it depends upon geometry of double bond in the starting material. For e.g., ❖ What would be the structure of the product when the following compound is heated? Ans: The above mentioned compound when heated would undergo Claisen Rearrangement and the structure of the product would be following. 3.5.11. Aromatic Claisen Rearrangement Aromatic Claisen Rearrangement involves rearrangement of allyl phenyl ether via [3,3]sigmatropic rearrangement. The intermediate generated in this case, very quickly undergoes tautomerization to form ortho substituted phenol. For e.g., There could be various probabilities with substitution at ortho, meta and para- positions. The substitution at meta position effects the regioselectivity of the rearrangement. When the ortho position is blocked of allyl phenyl ether, the reaction proceeds in the following manner: TRICK 101: When Para and ortho position filled, then no reaction will occur. 3.5.12. Ireland- Claisen Rearrangement The Ireland-Claisen Rearrangement is another approach of Claisen-Rearrangement wherein an allyl ester of carboxylic acid is used instead of allyl vinyl ether. The ester is further converted to silyl-stabilized enolate. The reaction begins with deprotonation of the α-hydrogen of the ester using LDA to form an enolate which then attacks TMSCl to stabilize the charge and produce LiCl salt. The two olefin groups are ideally positioned for a claisen rearrangement, which results in the formation of a carbonyl group. Deprotection of the TMS group with base and protonation then results in the final carboxylic acid product, proceeds in the following manner: 3.6. Group Transfer Reaction 1. Diimide and Related Reductions: Diimide is generated by oxidation of hydrazine and it is involved in the transfer of two groups. Concerted delivery of two hydrogen takes place with syn stereospecificity in Suprafacial way in the manner shown below: A few examples of Diimide and Related Reductions would be: 1. 2. 2. Ene Reaction: The amalgamation of double and triple bond to an alkene reactant carrying a transferable allylic hydrogen is known as ene-reactions. Ene reactions are favored when the hydrogen accepting reagent, the "enophile", is electrophilic. The ene reactions involve only one sigma bond (C – H) to break. For e.g., Additionally, Electron donating group on the exe and electron withdrawing group on the exophile speed up the reaction. The molecular orbital approach could be elucidated in the following manner: ❖ What would be the structure of the product for the following reactions? Ans: The structure of the product will be: ❖ What would be the structure of the expected product for the following reactions? Ans: The structure of the product will be: 3.6.1. Metallo-Ene Reactions The metallo-ene reactions are mechanistically similar when compared to classic enereactions. The transition state for metallo-ene reactions is a cyclic six membered transition state which is an amalgamation of allylic and alkene species which together undergo rearrangement. A C-C σ bond is formed between allylic and alkene species due to migration of allylic species to one of the terminus of alkene reactant. Allylic metal reagents (Example: metals Mg, Zn, Li, Ni, Pd, and Pt) takes part readily by migration of metal atom (instead of hydrogen atom) and result in formation of a new carbon – metal bond. ❖ What would be the structure of the product for the reaction mentioned below? Ans: The structure of the product will be ❖ Elucidate the structure of the product when the following compound is heated Ans: The structure of the expected product would be: 3.7. Cheletropic Process Cheletropic Process involve the generation of transition state with a cyclic array of atoms and an associated cyclic array of interacting orbitals which occurs as a result of reorganization of π and σ bonds. It is a concerted process in which two sigma bonds are made (or broken) which terminate at a single atom. For example, There is generation of singlet carbene followed by suprafacial addition as illustrated below. Also, one must know the fact that cheletropic reactions are stereospecific and reversible. 3.7.1. Molecular Orbital Description of Cheletropic Process A carbene is a neutral molecule containing a divalent carbon with six electrons in its valence shell. Due to this, carbenes are highly reactive electrophiles and generated as reaction intermediates. A singlet carbene contains an empty p orbital and a roughly sp2 hybrid orbital that has two electrons. Singlet carbenes add stereospecifically to alkenes, and alkene stereochemistry is retained. For [2+1] Cheletropic Process, the olefin reacts with singlet carbene, the molecular orbital approach has been shown below: The singlet carbene approach occurs in two different ways namely Linear Approach and Non-Linear Approach. a. Case I: Linear Approach: The Linear Approach involves 2 HOMO – LUMO interactions. In the linear approach, the electrons in the orbital of the small molecule are pointed directly at the π-system. The rotation will be disrotatory if the small molecule approaches linearly. b. Case II: Non-Linear Approach: SAMPLE 3.7.2. Elimination of N2 and CO Here shown below, are a few examples of elimination of CO and N2. In the second example, the carbonyl carbon gets eliminated as CO. The final outcome is making two new bonds to one atom and these are classical examples of Cheletropic Extrusions, wherein a stable molecule is eliminated. Infact, this provides entropic benefits of gaseous evolution and serves as the driving force for the reaction. 3.8. Ketene [2+2] Cycloaddition Reaction One of the few [2+2] allowed thermal cycloadditions allowed includes [2+2] Ketene Cycloadditions. The [2+2] Ketene addition is allowed as the transition state is stablised by the pi orbital of the Carbonyl stablising the electron deficient carbon on the alkene. The most nucleophillic atom on the diene adds to most electrophillic atom on ketene and is geometry of junction comes from cis double bond of Cyclopentadiene