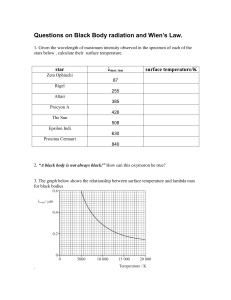
CHEM 330 SI worksheet 10 14 June 2021 1. What wavelength (in nanometres) is the peak intensity of the light coming from a star whose surface temperature is 11000 K? 2. If oven A has a λmax of 6 μ and oven B has a λmax of 7 μ , which oven is cooler? Show your working. 3. What is the total power per unit area emitted by a black body at a temperature of 1250 K? If the area of a blackbody is 1.00 cm2 what is the total power emitted? 4. Consider a photoelectric effect experiment as depicted in Figure II below. The incoming light with a frequency larger than Φ hits the Emitter. If you increase the frequency of the light and hold the intensity constant, does the number of electrons ejected per second from the metal surface (a) increase, (b) decrease, or (c) remain the same? Give your reasoning. 5. The work function for platinum metal is 8.0 × 10-10 J. a) Will a radiation of wavelength 200 nm be able to cause a photoelectric effect on the metal? b) If the electron is ejected, what would its velocity from the surface be? [10-10 J = 5042 cm-1 and mass of electron = 9.109 × 10-31 kg] 6. Find the wavelength of light emitted when a 1 x 10-27 g particle in a 5 Å one dimensional box goes from the n = 4 to n = 3 level. 7. An electron of mass 8.55 × 10-31 kg moves around its nucleus at speed of 3.51 × 106 ms-1. Estimate the uncertainty in position if the momentum is measured with an accuracy of 1%. 8. Which of the following molecules will show a rotational Raman spectrum and why? Methane, CH2Cl2, C2H2 and anthracene (C14H10). 9. The hydrogen fluoride molecule, 1H19F, in the gas phase has a single absorption band in the mid-infrared region of the electromagnetic spectrum at a wavenumber of 4141.3 1 𝑘 cm–1: Calculate the force constant, k, for hydrogen fluoride given that 𝑣 = 2𝜋 √𝜇 . [Masses: 19F = 18.9984 u, 1H = 1.0079 u] 10. The rotational spectrum of HF has lines that are 41.9 cm-1 apart. Calculate the bond length of this molecule. 11. State the relationship between van der Waals adsorption and activated adsorption in terms of energy of activation. 12. A monolayer of N2 molecule (effective area 0.165 nm2) is adsorbed on the surface of 1.00 g of Fe/Al2O3 catalyst at 77 K. Upon warming, nitrogen occupies 2.86 cm3 at 0 o C and 760 Torr. What is the surface area of the catalyst? 13. Reaction is now reported to occur via the Langmuir-Rideal mechanism as follows C2H6 (ads) + O2 (g) → H2O(g) + CO2 (g) With the aid of an equation, show the dependency of the reaction rate on the pressure of the reactants.