Electricity Unit: Static & Current Electricity, Power Generation
advertisement

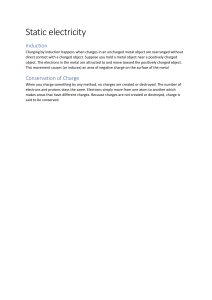
SCN1D/ ELECTRICITY UNIT Unit D: ATOMS , ELEMENTS AND COMPOUNDS OVERALL CURRICULUM EXPECTATIONS • E1. Assess some of the costs and benefits associated with the production of electrical energy from renewable and nonrenewable sources, and analyse how electrical efficiencies and savings can be achieved, through both the design of technological devices and practices in the home; • E2. Investigate, through inquiry, various aspects of electricity, including the properties of static and current electricity, and the quantitative relationships between potential difference, current, and resistance in electrical circuits; • E3. Demonstrate an understanding of the principles of static and current electricity. SPECIFIC EXPECTATIONS 11.1 WHAT IS STATIC ELECTRICITY? SNC1D RECALL: ATOMIC STRUCTURE Recall the 3 subatomic particles studied in chemistry: protons have a positive (+) charge electrons have a negative (-) charge neutrons have no (0) charge • The 2 subatomic particles contained in the nucleus are protons and neutrons. • Unlike protons and neutrons, electrons have the ability to move around and be removed from the atom. ELECTRIC CHARGE • If an atom has an unequal number of protons and electrons, it has an electric charge which will exert and electric force (repulsive or attractive). • A neutral object has an equal number of protons and electrons • A negatively charged object has more electrons than protons • A positively charged object has more protons that electrons. Examples: positive, negative, or neutral? ELECTRONS MOVE! • Objects may become electrically charged when electrons are transferred to or from another object. • Gaining electrons will make an object more negatively charged while losing electrons will make an object more positively charged. • Most of the objects we deal with on a daily basis are electrically neutral. When 2 neutral objects of different materials come in contact electrons can be transferred from one object to the other. Now both objects are charged. This creates STATIC ELECTRICITY: An imbalance of electric charge on the surface of an object. THE LAW OF ELECTRIC CHARGES • A charged object exerts an electric force, which can be either an attractive force (pulling together) or repulsive force (pushing apart). The Law of Electric Charges states: • OBJECTS OF LIKE CHARGES REPEL EACH OTHER • OBJECTS OF OPPOSITE CHARGES ATTRACT EACH OTHER THE LAW OF ELECTRIC CHARGES • Examples: Ebonite negatively charged Pith ball negatively charged Ebonite negatively charged Pith ball positively charged What happens when a charged object is brought towards a neutral object? • When a charged object is brought near a neutral object it causes (induces) the electrons to shift in position. • The induced movement of electrons in a neutral object by a nearby charged object is called an INDUCED CHARGED SEPARATION. The movement of electrons occurs according to the law of electric charges. Induced Charge Separation: a shift in the position of electrons in a neutral object that occurs when a charged object is brought near it. Detecting electric charges: pith ball electroscope • Pith is a plant material that can be used to test for the presence and type of electric charge on an object. • If the object is charged, the pith ball will be attracted to it. Detecting electric charges: metal leaf electroscope • The metal leaf electroscope is more sensitive to electric charge than a pith ball. The metal “leaves” are usually gold or aluminum. HOMEWORK • READ PG. 465- 470 • MAKE NOTES ON ELECTROSTATIC PAINT SPRAYERS IN THE DESIGNATED SECTION ON YOUR HANDOUT • ANSWER PG. 471 #2-7, 9 END OF WEEK 6 / CLASS 1