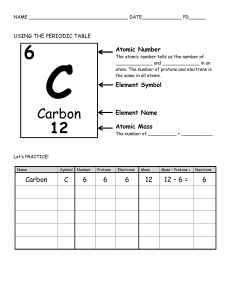
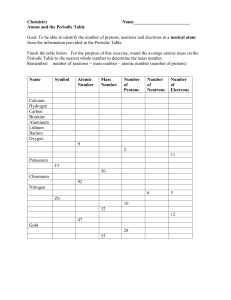
Build An Atom Worksheet: http://phet.colorado.edu/sims/html/build-an-atom/latest/build-an-atom_en.html Instructions: After completing each of the following steps, fill out the information in that row. Name of element Symbol Stable? (yes/no) Charge (-,0,+) Mass Number Atomic Number Number of Protons Name: Number of Neutrons Number of Electrons 1. After selecting the “Symbol” icon, drag a red proton to the yellow “X” (nucleus) 2. Drag a gray neutron to the nucleus. 3. Drag a blue electron to the first orbit. 4. Add another proton to the nucleus. 5. Add a neutron. 6. Add another neutron. 7. Add an electron. 8. Add another electron. 9. Add a proton. 10. Add another proton. Continue to add protons, neutrons, and electrons until you feel that you understand the relationships between the columns in the table above. Then circle the correct italicized term(s) in each sentence on the following page. © Copyright 2014 – All rights Reserved www.cpalms.org Created By Amy D. Alford 1. The addition or removal of (protons, neutrons, electrons) causes the element name to change. 2. Atomic number is equal to the number of (protons, neutrons, electrons). 3. Atomic mass is equal to the number of (protons, neutrons, electrons) plus the number of (protons, neutrons, electrons). 4. An atom becomes unstable when there are too many or not enough (protons, neutrons, electrons). 5. An atom becomes charged when the number of (protons, neutrons, electrons) does not equal the number of (protons, neutrons, electrons). 6. As you add protons, what pattern do you notice about the periodic table? A) The elements are ordered from right to left across the periodic table by increasing atomic number. B) The elements are ordered from left to right across the periodic table by increasing atomic number. C) The elements are ordered from right to left across the periodic table by increasing atomic mass. D) The elements are ordered from left to right across the periodic table by decreasing atomic mass. 7. (Protons, neutrons, electrons) are found only in the nucleus of an atom. (Choose two.) 8. (Protons, neutrons, electrons) are found only in the electron cloud. 9. Only (one, two, three) electrons could fit in the first inner orbit. 10.Bonus: How many electrons can fit in the second orbit? _________ Explore the games located in the “Game” icon on the black navigation bar at the bottom of the screen. © Copyright 2014 – All rights Reserved www.cpalms.org Created By Amy D. Alford