Chemistry Lab Manual: Boiling, Melting, Sublimation, Crystallization
advertisement

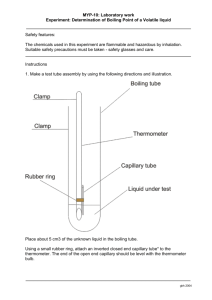
BOILING POINT DETERMINATION Reagents and Materials Needed thermometer tripod beaker isopropyl alcohol heating set-up oil bath stirring rod ethanol iron stand with ring oil iron clamp iron ring Procedure: 1. Prepare the set-up of apparatus for boiling point determination. 2. Place separately in 3 dry test tubes small amounts of ethanol reaching a height of 1 cm inside the test tube. 3. Place the test tube into the oil bath. Clamp it so that its bottom does not touch the bottom of the beaker. 4. Have the set-up approved by the instructor. 5. Heat with a moderate flame, and then gradually adjust to make it stronger so as to cause a rise in temperature of 1o to 2o per minute. Protect the flame from air drafts with a shield. 6. Stir the oil bath continuously to have a uniform heating. 7. Record the temperature at which the compound starts to boil (initial temperature) and the temperature (final temperature) at which it boils constantly. Note: Do not put off the flame when the liquid starts to boil. Continue heating until after the constant boiling temperature has been recorded. 8. Repeat the boiling point determinations with isopropyl alcohol. Note: Always start with a cool oil bath. Record the data in tabulated form. Compare the boiling points obtained and account for them in terms of structural effects. Guide Questions: 1. What is azeotropes or azeotropic mixtures? 2. What indications do the experimental results give regarding the purity of the samples used? 3. What may happen to the boiling point of a substance if there are impurities present? 4. What are the types of intermolecular attractive forces that hold liquid molecules together? 5. How do these intermolecular forces affect the boiling point of a liquid? thermometer beaker heating set-up salicylic acid MELTING POINT oil tripod capillary tube clamp stirring rod benzoic acid oil bath iron stand Procedure: 1. Prepare 3 melting point capillary tubes in the following manner: A. Heat the middle portion of a capillary tube (1mm diameter) and when soft enough, pull in opposite directions to completely seal and separate into two. B. Test if each prepared capillary tube is completely sealed on one end by blowing air on the open end, and feeling with the fingers if air comes out from the sealed end. If air comes out, the end should be heated further to completely seal it. 2. Introduce powdered samples of salicylic acid into three separate prepared capillary tube. This can be done by putting a small amount of the sample (about 3-4 mm in diameter) on a piece of clean dry paper, and pushing the open end of the capillary tube into the sample to scoop it up. The sample may be shaken down to the bottom by tapping on the side with the fingers, dropping the tube vertically into a hard surface several times, or the sample may be pushed to the bottom using a fine needle. Whichever method is done to introduce the sample, the column of solid should not be more than 1 cm, in the tube length and it should be tightly packed. 3. Prepare the set-up for melting point determination. 4. Fasten the capillary tube containing the sample to the thermometer with a string or a rubber band so that the sample is close to and on a level with the center of the thermometer bulb. Attach the thermometer in its place in the set-up where its bulb and the capillary tube (with sample) is centered in the beaker and submerged in the oil. 5. Have the set-up approve by your instructor. 6. Heat the oil bath with a moderate flame. Then gradually increase the size of the flame so as to cause a rise in temperature of 1°or 2° per minute. Protect the flame from air drafts with a shield. 7. Stir the oil bath continuously to have a uniform heating. 8. Record: A. The temperature at which melting begins, and B. The temperature when the entire 1 cm powdered sample in the capillary tube is completely melted. Note: do not confuse the melting point with softening point. 9. Repeat the same procedure by using benzoic acid. Note: Always start with a cool oil bath. Record the results obtained. Guide Questions: 1. What are the bonding forces that hold together the molecules in a crystal? Give examples of compounds with such types of bonding forces. 2. What is the effect of an impurity on the melting point of a substance? 3. How can melting point be used in identifying and/or characterizing unknown compounds? SUBLIMATION Reagents and Materials Needed round bottom flask salicylic acid beaker Heating set-up Oil bath distilled water Clamps Iron stand Tripod Iron ring Procedure: 1. Place 1 gram of salicylic acid in a clean, dry beaker. 2. Cover the beaker with a round-bottom flask with cold water. Note: Be careful so as not to spill the water and wet the sample in the beaker. 3. Heat gently with a low flame, or by placing on a water bath or hot plate. Observe the formation of the sublimate in pure crystalline form on the bottom of the cold flask and on the side walls inside the beaker. Note the temperature when the crystals started to form. 4. Cool the set-up completely and scrape off the crystals. Guide Questions: 1. What is sublimation? 2. What is the principle involved in sublimation? 3. Why do substances undergo sublimation? CRYSTALLIZATION Reagents and Materials Needed beaker benzoic acid stirring rod brown sugar boiling chips NaCl heating set-up methylene blue or congo red test tubes activated charcoal erlenmeyer flask 1% AgNO 3 ice water Procedure A 1. Prepare a mixture consisting of 1 g benzoic acid, a pinch of NaCl and 10 drops of methylene blue or congo red in a beaker. 2. Add 100 mL distilled water and heat with stirring. 3. When the benzoic acid has dissolved, add 0.5 g of activated charcoal, and continue heating with vigorous stirring. 4. Bring the solution to boiling and filter while still hot. 5. Collect 5 mL of the hot filtrate in a test tube labeled “slow cooling” and set it aside to cool slowly. 6. Collect another 5 mL of the hot filtrate in a second test tube and place in an ice bath. 7. Observe the filtrate and take note of the following: the color of the filtrate, the size of the crystals formed in rapid cooling and in slow cooling process. Record your observations. 8. Separate the crystals by filtration, washing with two separate portions of cold distilled water. Spread the crystals on a filter paper on a watch glass and allow to dry. (The crystals should be covered with a filter paper while drying) 9. Show the crystals to laboratory instructor. 10. Test for the completeness of the separation. Dissolve a small amount of the crystals in 1 mL hot distilled water in a test tube and add 1-2 drops of 1% AgNO3. Observe and record your result. Procedure B: 1. Dissolve 2 grams of brown sugar in 25 mL distilled water in a beaker. Note the color of the solution. 2. Heat to boiling and add 0.5 gram of activated charcoal with constant stirring. 3. Filter the solution while still hot. 4. Note the color of the filtrate. 5. Submit it to the laboratory instructor. Guide Questions: 1. What are the types of crystal? 2. What attractive forces bind molecular crystals together? Explain each briefly. 3. What is decolorization? 4. Why is it necessary to filter the solution while still hot? 5. What are the methods of inducing crystal formation from solution? 6. Crystals of what substance in the mixture were formed upon cooling? 7. What method produced bigger crystals, slow cooling or rapid cooling, why? 8. How is the completeness of separation of the benzoic acid crystals from NaCl known with the addition of AgNO 3 solution?