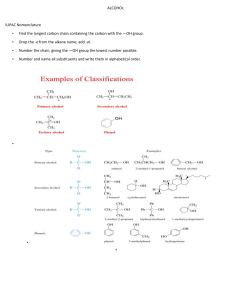
ALKENES • Also called olefins • Contain at least one carbon-carbon double bond (C=C) • General formula, CnH2n (n=2,3,…) • Classified as unsaturated hydrocarbons (compound with double or triple carbon-carbon bonds that enable them to add hydrogen atoms. • sp2-hybridized • For example: C2H4 - ethylene CH2 CH2 Naming Alkenes IUPAC RULES RULE 1. Select the longest continuous carbon chain that contains a double bond. This chain contains 6 carbon atoms RULE 2. Name this compound as you would an alkane, but change –ane to –ene for an alkene. This chain contains 8 carbon atoms This isthe theparent longest Name continuous octene. chain. compound Select it as the parent compound. RULE 3. Number the carbon chain of the parent compound starting with the end nearer to the double bond. Use the smaller of the two numbers on the double-bonded carbon to indicate the position of the double bond. Place this number in front of the alkene name. IUPAC RULES This end of the chain is closest to the double bond. Begin numbering here. IUPAC RULES The name of the parent compound is 1-octene. 4 3 2 1 5 6 7 8 RULE 4. Branched chains and other groups are treated as in naming alkanes. Name the substituent group, and designate its position on the parent chain with a number. IUPAC RULES The is attached to carbon 4. Thisethyl is an group ethyl group. 4 3 2 1 5 6 7 8 4-ethyl-1-octene • A compound with more than one double bond. - Two double bond: diene - Three double bond: triene - Four double bond: tetraene * Numbers are used to specify the locations of the double bonds. 1 2 3 CH2 C H 4 C CH2 H 7 6 CH3 C C H H IUPAC names: 1,3-butadiene new IUPAC names: buta-1,3-diene 1 2 8 3 7 4 6 5 5 IUPAC names: 1,3, 5, 7-cyclooctatetraene new IUPAC names: cycloocta-1,3,5,7-tetraene 4 3 C H C H 2 C H 1,3,5-heptatriene hepta-1,3,5-triene 1 CH2 ALKENES AS SUBSTITUENTS • Alkenes names as substituents are called alkenyl groups. • Can be named systematically as ethenyl, propenyl, etc. or by common names such as vinyl, ally, methylene and phenyl groups. CH2 methylene group (methylidene group) -CH=CH2 vinyl group (ethenyl group) -CH2-CH=CH2 allyl group (2-propenyl group) CH=CH2 CH2 CHCHCH2CH CH2 3-methylenecyclohexene 3-vinyl-1,5-hexadiene 3-vinylhexa-1,5-diene CYCLOALKENES • Contains C=C in the ring cyclopropene cyclobutene cyclohexene cyclopentene • Nomenclature of cycloalkenes: - Similar to that alkenes - Carbons atoms in the double bond are designated C1 and C2 6 5 4 CH3 1 2 3 1-methylcyclohexene 5 1 4 3 2 1,5-dimethylcyclopentene NOMENCLATURE OF cis-trans ISOMERS H3C CH2CH3 C H H3C C C H cis-2-pentene H H C CH2CH3 trans-2-pentene •cis – two particular atoms (or groups of atoms) are adjacent to each other •trans – the two atoms (or groups of atoms) are across from each other PHYSICAL PROPERTIES OF ALKENES • Boiling points and densities: -Most physical properties of alkenes are similar to those alkanes. -Example: the boiling points of 1-butene, cis-2-butene, trans-2-butene and n-butane are close to 0oC. - Densities of alkenes: around 0.6 or 0.7 g/cm3. -Boiling points of alkenes increase smoothly with molecular weight. -Increased branching leads to greater volatility and lower boiling points. • Polarity: - relatively nonpolar. -insoluble in water but soluble in non-polar solvents such as hexane, gasoline, halogenated solvents and ethers. - slightly more polar than alkanes because: i) electrons in the pi bond is more polarizable (contributing to instantaneous dipole moments). ii) the vinylic bonds tend to be slightly polar (contributing to a permanent dipole moment).