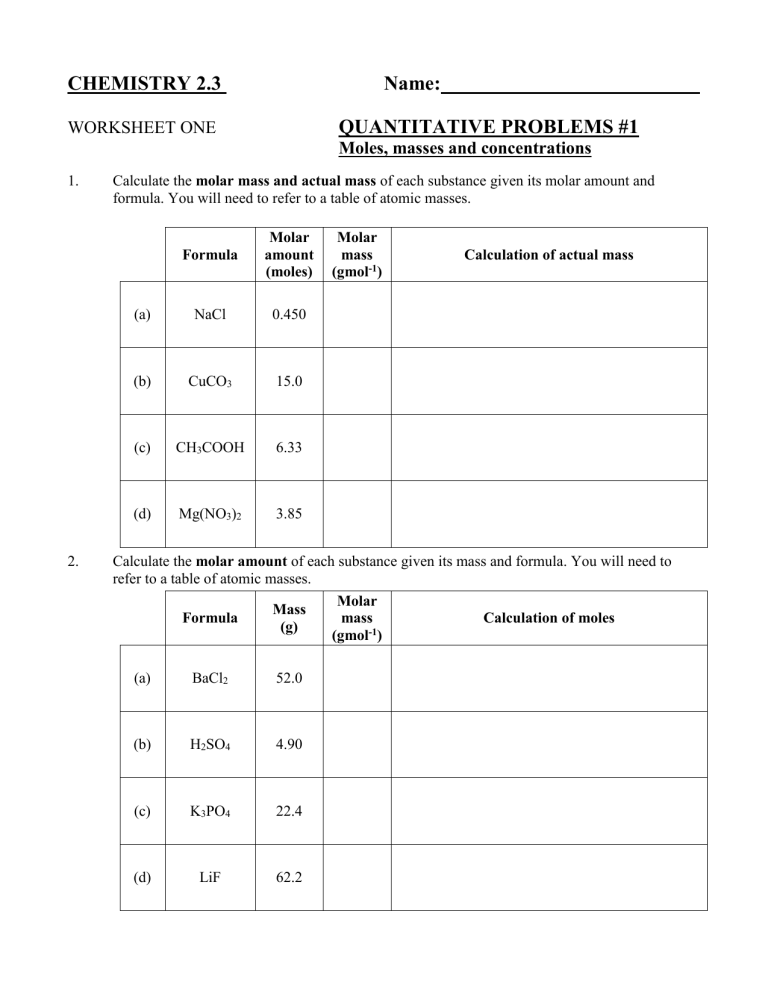
CHEMISTRY 2.3 Name: QUANTITATIVE PROBLEMS #1 WORKSHEET ONE Moles, masses and concentrations 1. 2. Calculate the molar mass and actual mass of each substance given its molar amount and formula. You will need to refer to a table of atomic masses. Formula Molar amount (moles) (a) NaCl 0.450 (b) CuCO3 15.0 (c) CH3COOH 6.33 (d) Mg(NO3)2 3.85 Molar mass (gmol-1) Calculation of actual mass Calculate the molar amount of each substance given its mass and formula. You will need to refer to a table of atomic masses. Molar Mass Formula mass Calculation of moles (g) (gmol-1) (a) BaCl2 52.0 (b) H2SO4 4.90 (c) K3PO4 22.4 (d) LiF 62.2 3. Calculate the concentrations of the following solutions in mol L-1. Solute Formula 4. Moles Solution of solute volume present (L) (a) NaOH 0.00200 0.120 (b) HCl 0.0150 0.500 (c) CH3COOH 2.25 10.2 (d) KOH 0.330 0.250 Concentration of aqueous solution Calculate the mass of solute present in the following solutions. You will need to refer to a table of atomic masses and calculate the molar masses first. Solute Formula Concn. of solution (mol L-1) Solution volume (mL) (a) NaCl 0.150 150 (b) H2SO4 2.00 250 (c) CuSO4 0.025 550 (d) NH4NO3 1.33 50 (e) Na2CO3 0.0067 800 Molar mass (gmol-1) Calculation of solute mass


