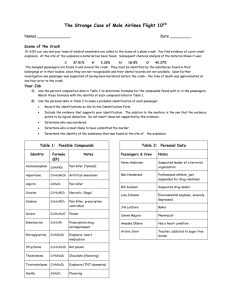
THE STRANGE CASE OF MOLE AIRLINES FLIGHT 1023 Scene of the Crash At 6:02 a.m. you and your team of medical examiners are called to the scene of a plane crash on Mendeleev Manor. You find evidence of a pre-crash explosion. At the site of the explosion a material has been found. Subsequent chemical analysis of the material shows it was: C N 37.01% 18.5% H O 2.22% 42.27% The mangled passengers are found in and around the crash. They must be identified by the substances found in their belongings or in their bodies, since they are not recognizable and their dental records are not available. Upon further investigation one passenger was suspected of having been murdered before the crash - the time of death was approximated at one hour prior to the crash. Your Job 1. Identify the purpose of your investigation and who you believe the murderer to be (hypothesis). Explain your procedure for investigation. Collect data from the tables below and use it with your calculations. 2. Use the percent composition data in Table 3 to calculate formulas for the compounds found with or in the passengers. Show all calculations in the data section of your lab report. Use Table 1 to identify each compound. 3. Use the personal data in Table 2 to make a probable identification of each passenger. ⮊ Create a data table to determine the identity of each victim. Include the evidence that supports your identification. ⮊ The solution to the mystery is the one that the evidence points to by logical deduction. Do not insert ideas not supported by the evidence. ⮊ As part of your conclusion you should explain who was murdered, who is the murderer, how they were killed, and identify what substance was found at the explosion site. Table 1: Possible Compounds *empirical formula instead of the molecular formula is used. Name Empirical Formula Notes Codeine C18H21NO3 Cocaine C17H21NO4 Pain killer, prescription controlled Narcotic, illegal Aspirin C9H8O4 Pain killer Aspartame C14H18N2O5 Artificial sweetener Vanilla C8H8O3 Flavoring Trinitrotoluene C7H5N3O6 Explosive (TNT-dynamite) Nitroglycerine C3H5N3O9 Explosive, heart medication Curare C40H44N4O Poison Theobromine C7H8N4O2 Chocolate (flavoring) Strychnine C21H22N2O2 Rat poison Dimetacrine C10H13N* Acetaminophen C8H9NO2 Prescription drug, antidepressant Pain killer (Tylenol) Table 2: Personal Passengers & Crew Redd D. Tocroak Polly Pillcounter Hoagie Bunn Archie Givengrades Threwit Allaway Ivanna Bedown Iselle Ubye Data Notes Has a heart condition Pharmacist Baker Teacher, addicted to sugar free drinks Professional athlete, just suspended for drug violations Environmental engineer, severely depressed Suspected drug dealer Table 3: Percent Composition Data of the Compounds Found in or with the Passengers’ Bodies Compound Analysis (% Composition) Passenger Location C (%) H (%) N (%) O (%) 1 67.30 6.99 4.62 21.09 2 63.14 5.31 -- 31.55 Face 46.66 4.48 31.10 17.76 Stomach 3 72.20 7.08 4.68 16.03 4 15.86 2.22 18.51 63.41 5 75.41 6.64 8.38 9.57 Pockets (2000 tablets) Blood and pockets Blood 37.01 2.22 18.50 42.26 Pockets 6 57.12 6.18 9.52 27.18 Blood 7 75.41 6.64 8.38 9.57 Pockets 81.57 8.92 9.515 -- Pockets 59.99 4.48 -- 35.52 Pocket 63.55 6.01 9.27 21.17 Pocket 8 Blood THE STRANGE CASE OF MOLE AIRLINES FLIGHT 1023 Norm Anderson Suspected leader of a terrorist organization Steps for solving (Note: The underlined words are the sections you should have in your final writeup.) 1. Read through the information you are given and write a purpose (what are you trying to do) and a hypothesis (what do you think happened - This is clearly an educated guess because you don’t know yet). I believe that trinitrotoluene is the substance found on the crash site due to the percentages of each substance matching with the ones at the crash site. 2. Write a BRIEF procedure for determining the identity of the 12 substances as well as the identity of the 8 passengers. 3. Use the percent composition data in table 3 to find empirical formulas of each of the 12 compounds, plus the compound at the top of the sheet that was found at the crash site. Show ALL of your calculations in an organized way. (Note: if a box is labelled " -- " it means that that element is not present in that compound.) 4. Create a data table in your lab notebook like the table below. Leave yourself room to write multiple compounds for some passengers and room to write explanations in the far right column. Passenger Empirical Formula of Compound(s) Name of Compound(s) Name of Person Explanation (how did you identify this passenger?) Vanilla, chocolate Hoagie Bun We identified Hoagie Bun because vanilla and chocolate are commonly used by bakers when making sweets and pastries. We identified Redd D. Tocroak because this passenger had a heart condition which indicates that they take heart medication. Given that Polly is a pharmacist, it can be concluded that they has the codeine because that is a prescription controlled drug and they have a large quantity of these tablets. Archie is addicted to sugar free drinks so given that, It makes sense that they would also have the artificial sweetener to aid with their addiction 1 C8H8O3 , C7H8N4O2 2 C3H5N3O9 Nitroglycerine 3 C18H21NO3 Codeine Polly Pillcounte r 4 C14H18N2O5 Archie Givengrad es 5 C17H21NO4 (Aspartame)Arti ficial sweetener Cocaine 6 C10H13N C21H22N2O2 Dimetacrine 7 C9H8O4C8H9NO2 Pain killer; Pain killer (tylenol) Iselle Ubye 8 C7H5N3O6 C40H44N4O Explosive (TNT-dynamite) ;Poison Norm Anderson Redd D. Tocroak Threwit Allaway Ivanna Bedown Due to Threwit Allaway’s history for doing illegal drugs, we can safely assume that he would be the passenger that has brought the cocaine onto the plane. Ivanna suffers from severe depression which rationalises her possession of the antidepressant Dimetacrine Iselle Ubye is a suspected drug dealer that has boarded the plane. We have reason to believe that she is the owner of the Asprin and the Acetaminophen due to the suspicion of her dealing drugs. Norm Anderson is likely the leader to a terrorist organization. This could be used as evidence that he would have brought TNT onto the plane. 5. After calculating the empirical formulas of each of the 12 compounds, compare the empirical formula you find for each substance to the empirical formulas listed in table 1. Use this information to identify the 12 compounds found in/on each passenger and add the empirical formulas and names of the compounds to the table in your lab notebook. 6. Use the information in table 3 and your data table to identify each passenger based on the notes you have about that passenger. Make sure you pay attention to where each compound was found on each passenger – whether they were THE STRANGE CASE OF MOLE AIRLINES FLIGHT 1023 carrying it with them or had it in their blood/stomach. This can help you narrow down the possibilities. Fill in the name of the person and the explanation for how you identified that person in the table in your lab notebook. 7. Identify the compound found at the crash site using the empirical formula you calculated. Use the information in your data table to identify which passenger was murdered before the plane crash and who the murderer was. Write a conclusion paragraph that explains what happened and how you know (Who was murdered? Who was the murderer? How was the person killed?What substance caused the plane crash? How did the data you were given help you answer all of these questions?) Some available resources to present your final writeup Microsoft Publisher Piktochart Google slides Canva