Mathematical Modeling of Mycophenolate Mofetil (MMF): antiproliferative agent of target
cells for HIV Cure
Introduction
Challenges of Antiretroviral Therapy for HIV Treatment
Development of baseline Density-dependent HIV Active Infection Model
1. Should proliferation terms include a carrying
capacity?
2. Should MMF affect proliferation, infectivity
of virus towards susceptible cells, or both?
3. Should actively infected cells proliferate, and
if so, at a rate equivalent to that of
susceptible cells?
Kulpa, D.A. et al. Journal of Virus Eradication (2015)
• Antiretroviral therapy (ART) suppresses HIV replication
• Daily challenges: Accessibility, affordability, and toleration
• As of 2017, <60% of patients have daily ART access and <50%
of patients achieve viral suppression.
Antiproliferative Therapy for HIV Reservoir Depletion/Cure
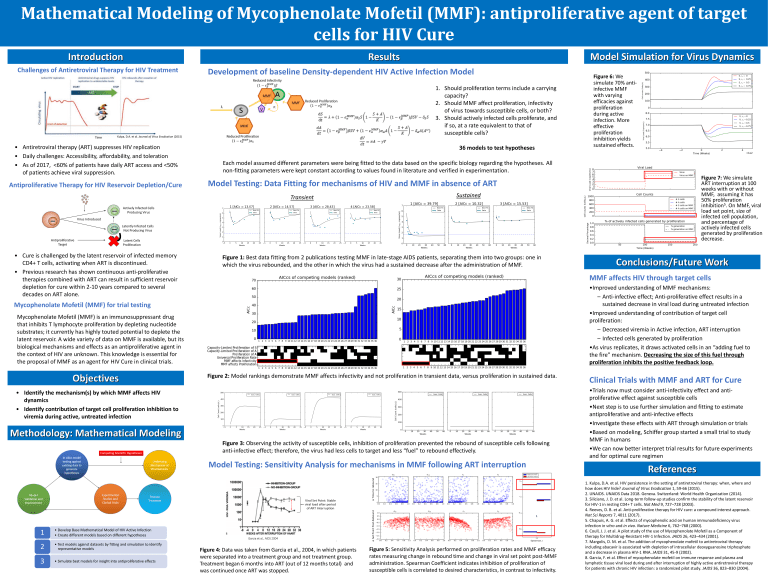
Model Simulation for Virus Dynamics
Results
36 models to test hypotheses
Figure 6: We
simulate 70% antiinfective MMF
with varying
efficacies against
proliferation
during active
infection. More
effective
proliferation
inhibition yields
sustained effects.
Each model assumed different parameters were being fitted to the data based on the specific biology regarding the hypotheses. All
non-fitting parameters were kept constant according to values found in literature and verified in experimentation.
Figure 7: We simulate
ART interruption at 100
weeks with or without
MMF, assuming it has
50% proliferation
inhibition5. On MMF, viral
load set point, size of
infected cell population,
and percentage of
actively infected cells
generated by proliferation
decrease.
Model Testing: Data Fitting for mechanisms of HIV and MMF in absence of ART
Transient
Sustained
Actively Infected Cells
Producing Virus
Virus Introduced
Latently Infected Cells
Not Producing Virus
Antiproliferative
Target
Latent Cells
Proliferation
• Cure is challenged by the latent reservoir of infected memory
CD4+ T cells, activating when ART is discontinued.
• Previous research has shown continuous anti-proliferative
therapies combined with ART can result in sufficient reservoir
depletion for cure within 2-10 years compared to several
decades on ART alone.
Figure 1: Best data fitting from 2 publications testing MMF in late-stage AIDS patients, separating them into two groups: one in
which the virus rebounded, and the other in which the virus had a sustained decrease after the administration of MMF.
MMF affects HIV through target cells
•Improved understanding of MMF mechanisms:
– Anti-infective effect; Anti-proliferative effect results in a
sustained decrease in viral load during untreated infection
•Improved understanding of contribution of target cell
proliferation:
– Decreased viremia in Active infection, ART interruption
– Infected cells generated by proliferation
•As virus replicates, it draws activated cells in an “adding fuel to
the fire” mechanism. Decreasing the size of this fuel through
proliferation inhibits the positive feedback loop.
Mycophenolate Mofetil (MMF) for trial testing
Mycophenolate Mofetil (MMF) is an immunosuppressant drug
that inhibits T lymphocyte proliferation by depleting nucleotide
substrates; it currently has highly touted potential to deplete the
latent reservoir. A wide variety of data on MMF is available, but its
biological mechanisms and effects as an antiproliferative agent in
the context of HIV are unknown. This knowledge is essential for
the proposal of MMF as an agent for HIV Cure in clinical trials.
Objectives
Figure 2: Model rankings demonstrate MMF affects infectivity and not proliferation in transient data, versus proliferation in sustained data.
• Identify the mechanism(s) by which MMF affects HIV
dynamics
• Identify contribution of target cell proliferation inhibition to
viremia during active, untreated infection
Methodology: Mathematical Modeling
Figure 3: Observing the activity of susceptible cells, inhibition of proliferation prevented the rebound of susceptible cells following
anti-infective effect; therefore, the virus had less cells to target and less “fuel” to rebound effectively.
Model Testing: Sensitivity Analysis for mechanisms in MMF following ART interruption
1
• Develop Base Mathematical Model of HIV Active Infection
• Create different models based on different hypotheses
2
• Test models against datasets by fitting and simulation to identify
representative models
3
• Simulate best models for insight into antiproliferative effects
Conclusions/Future Work
Figure 4: Data was taken from Garcia et al., 2004, in which patients
were separated into a treatment group and not treatment group.
Treatment began 6 months into ART (out of 12 months total) and
was continued once ART was stopped.
Figure 5: Sensitivity Analysis performed on proliferation rates and MMF efficacy
rates measuring change in rebound time and change in viral set point post-MMF
administration. Spearman Coefficient indicates inhibition of proliferation of
susceptible cells is correlated to desired characteristics, in contrast to infectivity.
Clinical Trials with MMF and ART for Cure
•Trials now must consider anti-infectivity effect and antiproliferative effect against susceptible cells
•Next step is to use further simulation and fitting to estimate
antiproliferative and anti-infective effects
•Investigate these effects with ART through simulation or trials
•Based on modeling, Schiffer group started a small trial to study
MMF in humans
•We can now better interpret trial results for future experiments
and for optimal cure regimen
References
1. Kulpa, D.A. et al. HIV persistence in the setting of antiretroviral therapy: when, where and
how does HIV hide? Journal of Virus Eradication 1, 59-66 (2015).
2. UNAIDS. UNAIDS Data 2018. Geneva. Switzerland: World Health Organization (2014).
3. Siliciano, J. D. et al. Long-term follow-up studies confirm the stability of the latent reservoir
for HIV-1 in resting CD4+ T cells. Nat Med 9, 727–728 (2003).
4. Reeves, D. B. et al. Anti-proliferative therapy for HIV cure: a compound interest approach.
Nat Sci Reports 7, 4011 (2017).
5. Chapuis, A. G. et al. Effects of mycophenolic acid on human immunodeficiency virus
infection in vitro and in vivo. Nature Medicine 6, 762–768 (2000).
6. Coull, J. J. et al. A pilot study of the use of Mycophenolate Mofetil as a Component of
therapy for Multidrug-Resistant HIV-1 Infection. JAIDS 26, 423–434 (2001).
7. Margolis, D. M. et al. The addition of mycophenolate mofetil to antiretroviral therapy
including abacavir is associated with depletion of intracellular deoxyguanosine triphosphate
and a decrease in plasma HIV-1 RNA. JAIDS 31, 45-9 (2002).
8. Garcia, F. et al. Effect of mycophenolate mofetil on immune response and plasma and
lymphatic tissue viral load during and after interruption of highly active antiretroviral therapy
for patients with chronic HIV infection: a randomized pilot study. JAIDS 36, 823–830 (2004).



