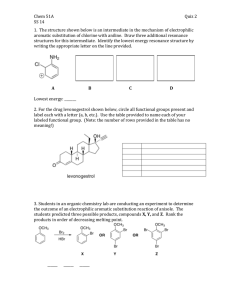
Chemistry of Benzene: Electrophilic Aromatic Substitution Michael Dann A. Superio Contents Electrophilic Aromatic Substitution: Bromination Other Aromatic Substitution Alkylation and Acylation of Aromatic Rings: The Friedel-Crafts Reaction Substituent Effects on Aromatic Rings An Explanation of Substituent Effects Trisubstituted Benzenes: Additivity of Effects Nucleophilic Aromatic Substitution Oxidation of Aromatic Compounds Reduction of Aromatic Compounds Synthesis of Polysubstituted Benzenes Electrophilic Aromatic Substitution Reaction: Bromination Electrophilic Aromatic Substitution Reaction: Bromination Electrophilic Aromatic Substitution Reaction: Bromination • For bromination to take place, a catalyst (FeBr3), is used. The catalyst makes Br2 more electrophilic by polarizing it to FeBr4-Br+ species that reacts as if it were Br+. Other Aromatic Substitution Reactions Aromatic Fluorination, Chlorination, and Iodination • Fluorine (F2) is too reactive; other sources of F+ is used where it is bonded to a more stable N+. • F-TEDA-BF4 (Selectfluor) Aromatic Fluorination, Chlorination, and Iodination • Chlorination happens in the presence of FeCl3 catalyst to yield chlorobenzenes. Aromatic Fluorination, Chlorination, and Iodination • Iodine is unreactive toward aromatic rings: needs oxidizing agents (hydrogen peroxide, copper salt such as CuCl2). Aromatic Fluorination, Chlorination, and Iodination • Aromatic halogenation also occur in the biosynthesis of many naturally occurring molecules. Best known among humans happens in the thyroid gland. Aromatic Nitration • Addition of a nitrate occurs when aromatic rings are reacted with a mixture of concentrated nitric and sulfuric acids. Aromatic Nitration • Electrophilic nitration of aromatic rings do not occur in nature but is often induced in laboratory. • Nitro-substituted aromatic rings can be nitrated (NO2 → NH2) using iron, tin, or SnCl2 to yield arylamines (ArNH2): important in the synthesis of dyes and pharmaceutical agents. Aromatic Sulfonation • Aromatic rings can be sulfonated by reaction with fuming sulfuric acid (H2SO4 + SO3) • Substitution occurs just like in bromination. • Sulfonation is reversible • Sulfonation is favored in strong acid • Desulfonation is favored in hot, diluted aqueous acid Aromatic Sulfonation • Does not occur naturally but is used in the preparation of dyes and pharmaceutical agents. • Sulfa drugs (sulfanilamide) were among the first clinically useful antibiotics. Aromatic Hydroxylation • Direct hydroxylation of an aromatic ring to yield a hydroxybenzene (phenol) is difficult and rarely done in laboratory; occurs frequently in biological pathways though. • By analogy with other aromatic substitution, we expect an electrophilic oxygen species is needed (OH+ equivalent) is needed for hydroxylation. Alkylation and Acylation: The Friedel-Crafts Reaction Alkylation and Acylation: The Friedel-Crafts Reaction • Alkylation: the addition of an alkyl group (-R) • Acylation: the addiction of an acyl group (-COR) • Among the most useful electrophilic aromatic substitution reaction in the laboratory • Carried out by treating the aromatic compound with an alkyl chloride in the presence of AlCl3 to generate a carbocation electrophile. Alkylation and Acylation: The Friedel-Crafts Reaction • Has several limitations • Only alkyl halides can be used (aromatic halides and vinyl halides cannot be used because they are too high in energy) • Do not succeed on aromatic rings with strong electron-withdrawing group (carbonyl) or by a basic amino group that can be protonated • Difficult to stop the reaction after a single substitution; goal product cannot be easily taken. Alkylation and Acylation: The Friedel-Crafts Reaction • Yet a final limitation: skeletal rearrangement of the alkyl carbocation sometimes occur. • Means that the product is a mix of isomers of the expected product • Occurs either by hydride shift or alkyl shift Alkylation and Acylation: The Friedel-Crafts Reaction • Recall, acylation: aromatic ring is added with a carbonyl-containing group (-COR) in the presence of AlCl3. Alkylation and Acylation: The Friedel-Crafts Reaction Alkylation and Acylation: The Friedel-Crafts Reaction • Aromatic alkylation occur in numerous biological pathways. • However, no AlCl3 is involved. • Carbocation electrophile is formed by dissociation of an organodiphosphate, usually assisted by Mg2+. Substituent Effect in Substituted Aromatic Rings Substituent Effect in Substituted Aromatic Rings • When benzene is subjected to a reaction, only one product is formed (mostly). What would happen if that benzene has a substituent attached to it? • Substituent has two effects: • Affect the reactivity: makes the ring more reactive, or more non-reactive Substituent Effect in Substituted Aromatic Rings • When benzene is subjected to a reaction, only one product is formed (mostly). What would happen if that benzene has a substituent attached to it? • Substituent has two effects: • Affect the orientation: the three orientation (ortho, meta, para) are not formed in equal amounts. Substituent nature and position determine the position of the second substitution. Substituent Effect in Substituted Aromatic Rings • Substituents can be divided into three groups: • Ortho- and para-directing activators (mostly activating) • Ortho- and para-directing deactivators • Meta-directing deactivators (mostly deactivating) Substituent Effect in Substituted Aromatic Rings • Predict the major product of the sulfonation of toluene. + SO3 H2SO4 ??? Quick Quiz Predict the major products of the following reactions • • • • Nitration of bromobenzene Bromination of nitrobenzene Chlorination of phenol Bromination of aniline An Explanation of Substituent Effects An Explanation of Substituent Effects • What makes a group activating or deactivating? • Activating: they donate electrons • Deactivating: they withdraw electrons • Withdrawal or donation of electrons is controlled by an interplay of inductive and resonance effects • Inductive effect: withdrawal or donation of σ bonds due to electronegativity An Explanation of Substituent Effects • What makes a group activating or deactivating? • Activating: they donate electrons • Deactivating: they withdraw electrons • Withdrawal or donation of electrons is controlled by an interplay of inductive and resonance effects • Resonance effect: withdrawal or donation through π bond due to overlap of p orbitals An Explanation of Substituent Effects • What makes a group activating or deactivating? • Activating: they donate electrons • Deactivating: they withdraw electrons • Withdrawal or donation of electrons is controlled by an interplay of inductive and resonance effects • Resonance effect: withdrawal or donation through π bond due to overlap of p orbitals Trisubstituted Benzenes: Additivity of Effects Trisubstituted Benzenes: Additivity of Effects • What if the ring contains two substituents instead of one? • Governed by the same inductive and resonance effects • Difference to monosubstituted benzene: the two substituents have an additive effect • Three rules: • If the directing effects of the two groups reinforce each other, the situation is straightforward. • If the directing effects of the two groups oppose each other, the more powerful activating group has the dominant influence, but mixtures of products are often formed. • Further substitution rarely occurs between the two groups in a meta-disubstituted compound because this site is too hindered. Aromatic rings with three adjacent substituents must therefore be prepared by some other route, such as by substitution of an ortho-disubstituted compound. Trisubstituted Benzenes: Additivity of Effects • If the directing effects of the two groups reinforce each other, the situation is straightforward. Trisubstituted Benzenes: Additivity of Effects • If the directing effects of the two groups oppose each other, the more powerful activating group has the dominant influence, but mixtures of products are often formed. Trisubstituted Benzenes: Additivity of Effects • Further substitution rarely occurs between the two groups in a metadisubstituted compound because this site is too hindered. Aromatic rings with three adjacent substituents must therefore be prepared by some other route, such as by substitution of an ortho-disubstituted compound. Trisubstituted Benzenes: Additivity of Effects • What product would you expect from bromination of pmethylbenzoic acid? + Br2 Fe2Br3 ??? Quick Quiz Part 2 • At what position would you expect electrophilic substitution to occur in each of the following substances? Quick Quiz Part 2 • Show the major product(s) from the reaction of the following substances with (1) CH3CH2Cl, AlCl3 and (2) HNO3, H2SO4 Nucleophilic Aromatic Substitution Nucleophilic Aromatic Substitution • Although aromatic substitution usually occur by electrophilic mechanism, aryl halides that have electron-withdrawing substituents can also undergo nucleophilic substitution reaction. Nucleophilic Aromatic Substitution • Much less common than electrophilic substitution; have certain uses • Used in detection of free amino acids • Can be used in amino acid sequencing Nucleophilic Aromatic Substitution • Only occurs if aromatic ring has an electronwithdrawing substituent in a position ortho or para to the leaving group to stabilize the anion intermediate through resonance. Nucleophilic Aromatic Substitution • Difference between electrophilic and nucleophilic substitution • ES: favored by electron-donating substituent; NS: favored by electronwithdrawing substituent • ES: meta-directing substituents; NS: ortho-para directing substituents • ES: replace hydrogen on the ring; NS: replace a leaving group (usually halide) Oxidation of Aromatic Compounds Oxidation of Aromatic Compounds • Despite the unsaturation, benzene ring is unreactive to strong oxidizing agents such as KMnO4 and Na2CrO7. • However, presence of aromatic rings has effect on alkyl side chains. • Alkyl side chains rapidly react with oxidizing agents and convert to carbonyl groups (-COOH). Alkylbenzene becomes benzoic acid. Oxidation of Aromatic Compounds • Analogous side-chain oxidation also happens in biological pathways. Norepinephrine is biosynthesized from dopamine by benzylic hydroxylation reaction. Reduction of Aromatic Compounds Reduction of Aromatic Compounds • Reduction = addition of hydrogen (hydrogenation) • Benzene is generally inert to hydrogenation under typical alkene conditions. • Because of this, it is possible to selectively reduce an alkene double bond in the presence of an aromatic ring. Reduction of Aromatic Compounds • To hydrogenate an aromatic ring, it is necessary to use a platinum catalyst with hydrogen gas at high pressure or use a more effective catalyst such as rhodium on carbon (Rh/C). Aromatic rings are converted into cycloalkanes. Synthesis of Polysubstituted Benzenes Synthesis of Polysubstituted Benzenes • How do we synthesize an aromatic compound with multiple substituent? • You need: • Analytical ability, knowledge of the use and limitations of organic reactions • Know which reaction to use and when to use it • Best way to learn organic chemistry • Challenges your ability to think critically and analyze situations to plan properly, a good skill that is very much needed in Dentistry. Synthesizing a Polysubstituted Benzene • Synthesize 4-bromo-2-nitrotoluene from benzene • Strategy • Draw the target molecule, identify the substituents, and recall how each group can be introduced into the ring separately. Then plan retrosynthetically (go backwards from the product to its reactants). The three substituents on the ring are a bromine, a methyl group, and a nitro group. A bromine can be introduced by bromination with Br2/FeBr3, a methyl group can be introduced by Friedel–Crafts alkylation with CH3Cl/AlCl3, and a nitro group can be introduced by nitration with HNO3/H2SO4. Synthesizing a Polysubstituted Benzene • Synthesize 4-chloro-2-propylbenzenesulfonic acid from benzene • Strategy • Draw the target molecule, identify the substituents, and recall how each group can be introduced into the ring separately. Then plan retrosynthetically (go backwards from the product to its reactants) The three substituents on the ring are a chlorine, a propyl group, and a sulfonic acid group. A chlorine can be introduced by chlorination with Cl2/FeCl3, a propyl group can be introduced by Friedel–Crafts acylation with CH3CH2COCl/AlCl3 followed by reduction with H2/Pd, and a sulfonic acid group can be introduced by sulfonation with SO3/H2SO4. Quick Quiz • How might you synthesize the following substances from benzene? a. m-chloronitrobenzene b. m-chloroethylbenzene c. 4-chloro-1-nitro-2-propylbenzene d. 3-bromo-2-methylbenzenesulfonic acid