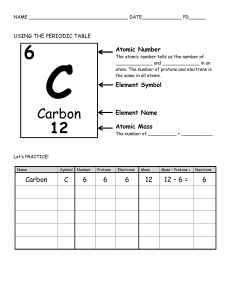
How to Read the Periodic Table The Periodic table is designed to help you predict what an element's physical and chemical properties are. You can also predict what elements will bond with each other. First, let's look at the columns and rows of the periodic table. Groups or Families The 18 vertical (up & down) columns of the periodic table are called groups or families. Elements in the same group or family have similar but not identical characteristics. You can know properties of a certain element by knowing which group it belongs to. Periods The horizontal (side to side) rows of the periodic table are called periods. Elements in a period are not alike in properties. As a rule, the first element in a period is usually an active solid, and the last element in a period is always an inactive gas. Atomic size decreases from left to right across a period, but atomic mass increases from left to right across a period. Atoms on the left of the period, therefore, are usually larger and more lightweight than the smaller, heavier atoms on the right of the period. Think Inside the Box When you look at the periodic table, you should notice that each box represents a different element, and each box contains information about the element, including its name, symbol, atomic number, and atomic mass. Look at the sample box below for a description of each of these pieces of information. 6 C Carbon 12.011 The top number is the atomic number. Every element has its own atomic number which tells how many protons are in one atom of that element. Since no two elements have the same atomic number, no two elements have the same number of protons. The large letter is the element's symbol, and just below that is the element's name. Each element has its own unique symbol and name. Below the name is the element's atomic mass. The atomic mass essentially gives you an estimate of how massive one atom of that element is. Elements vs. Compounds Elements are substances that cannot be separated into simpler substances. The smallest particles of matter are called atoms. Remember the carrot from the other chapter. If you continue to chop a carrot into smaller and smaller pieces, eventually you would reach a point where you could not cut up the carrot anymore, but still have carrot. You would then have molecules of carrot. The same applies to elements. If you continually cut up a piece of aluminum, you will reach a point that you could no longer divide it. These are aluminum atoms. An atom is the smallest particle of an element that has the properties of that element. Some properties of aluminum are: shiny, silver colored, fragile, and thin. Each element has its own type of properties. Chemists use symbols to represent elements. A symbol is a letter or picture used to represent something. Chemists use letters to represent elements. The symbol for aluminum is Al. The symbol for oxygen is O. Compounds A compound is a substance formed when two or more elements are chemically joined. Because compounds are made more than one element it is possible to break them down into their separate elements. Water, salt, and sugar are examples of compounds. Salt is made up of the elements sodium and chloride. Water is made up of the elements hydrogen and oxygen. Sugar is a combination of hydrogen, carbon, and oxygen. When the elements are joined, the atoms lose their individual properties and have different properties from the elements they are composed of. For example, carbon is basically charcoal and hydrogen and oxygen are clear gases. When they chemically combine in the right way they form sugar… which is nothing like its separate ingredients!