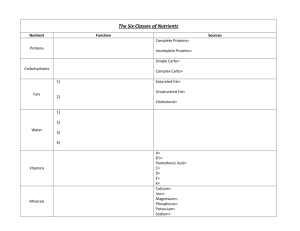
Vitamin B5 (Pantothenic Acid) HISTORY AND DEVELOPMENT • • Pantothenic Acid was isolated in 1938 by Dr. R. J. Williams and synthesized by other investigators in 1940. Although its vitamin nature was demonstrated by its ability to prevent certain deficiencies in animals, little interest was shown in this vitamin until about a decade later. In 1946, Lipmann and his associates showed that coenzyme A was essential for acetylation reactions in the body, and in 1950 reports from this same laboratory showed pantothenic acid to be a constituent of coenzyme A. HISTORY AND DEVELOPMENT • • The name for this vitamin is derived from the Greek word panthos, meaning “everywhere”. The universal distribution of this vitamin in biologic materials suggests the key role that it plays in metabolism. CHEMISTRY AND CHARACTERISTICS • PANTOTHENIC Acid, as the free acid, is an unstable, viscous yellow oil, soluble in water. • Commercially it is available as the sodium or calcium salt, which is slightly sweet, water soluble and quite soluble. • There is little loss of the vitamin with ordinary cooking procedures, except in acid and alkaline solutions. • The pantothenic acid content of tissues and foods is determined by microbiologic, chemical or radioimmunoassay methods; values are expressed in milligrams or micrograms. Body Distribution • Pantothenic acid along with 4’-phosphopantothenate and pantothenic may be found in the body’s cells. • Free Pantothenic acid is found in plasma, however, higher concentrations are found intracellularly: • Mainly in RBC’s • Most ingested pantothenic acid is used to synthesize or resynthesize CoA • Found in fairly high concentrations in the: • • • • • Liver Adrenal gland Kidney Brain Heart DIGESTION, ABSORPTION AND TRANSPORT • • 85% of the pantothenic acid found in foods is bound to CoA. • During digestion, CoA is hydrolyzed in the lumen to pantothenic acid Via phosphatases and pyrophosphatases. Free pantothenic acid can then be absorbed (mainly in the jejunum) • • High concentrations by passive diffusion Low concentrations via a Na+ dependent transport system • 40 to 61% of pantothenic acid is absorbed. • From the intestinal cell, it will enter the portal blood for transport to body cells. • Pantothenic acid is found free in blood plasma/serum; however, higher concentrations are found intracellularly (specifically within red blood cells) than extracellularly (in plasma/serum). EXCRETION • Pantothenic acid does not appear to undergo metabolism prior to excretion. • Pantothenic acid is excreted intact primarily in the urine, with only small amounts excreted in the feces. • No metabolites of the vitamin have been identified in the urine or feces. • Urinary excretion of the vitamin usually ranges from about 2 to 7 mg/day. FUNCTIONS OF PANTOTHENIC ACID • Pantothenic acid functions in the body as a component of COENZYME A (CoA) and as a prosthetic group on the acyl carrier protein. • CoA is a complex molecule consisting of a sulfur containing • compound, adenine, ribose, phosphoric acid and pantothenic acid. The sulfur linkage is highly reactive. • The part of the acyl carrier protein containing pantothenic acid has a structure similar to part of CoA. • CoA functions in reactions that accept or remove the acetyl group (-CH3CO). One of these reactions is the formation of acetyl choline, a substance of importance in the transmission of the nerve impulse. FUNCTIONS OF PANTOTHENIC ACID • CoA participates in the oxidation of pyruvate, α-keto-glutarate and fatty acids. • CoA reacts with pyruvic acid to form acetyl CoA, which, in turn, combines with oxaloacetate to form citrate thus initiating the TCA cycle for the release of energy. • CoA is also involved in the synthesis of cholesterol and other sterols, and porphyrin in the hemoglobin molecule. The acyl carrier protein has an essential role in fatty acid synthesis. • CoA is synthesized in all cells and apparently does not cross cell membranes. Liver, kidney, brain, adrenal and heart tissues, being metabolically active, contain high concentrations. PANTOTHENIC ACID RECOMMENDATIONS • An Adequate Intake (AI) for pantothenic acid has been set. It reflects the amount needed to replace daily losses. RECOMMENDED DIETARY ALLOWANCES (ADEQUATE INTAKE (MG/DAY) • Infants (Birth -1 year) • • • • Infants (0-0.5 yr): 1.7 mg/day • Infants (0.5-1 yr): 1.8 mg/day Children (1-8 year) • Children (1-3 yr): 2 mg/day • Children (4-8 yr): 3 mg/day Males • 9-13 years: 4 mg/day • 14-70 years: 5mg/day • >70 years: 5 mg/day Females • 14-18 years: 4 mg/day • 19-70 years: 5 mg/day • >70 years: 5 mg/day • Pregnancy: 6 mg/day • Lactation: 7 mg/day FOOD SOURCES • Pantothenic acid is widespread in foods, and typical diets seem to provide adequate intakes. • Beef, poultry, liver, egg yolk, whole grains, potatoes, tomatoes, and broccoli are particularly good sources. • Losses of pantothenic acid during food production can be substantial because it is readily destroyed by the freezing, canning, and refining processes. PANTOTHENIC ACID DEFICIENCY • Pantothenic acid deficiency is rare. • Its symptoms involve a general failure of all the body’s systems and include fatigue, GI distress, and neurological disturbances. • The “burning feet” syndrome that affected prisoners of war in Asia during World War II is thought to have been caused by pantothenic acid deficiency. • “Burning feet syndrome” is characterized by numbness of the toes and a • • sensation of burning in the feet. The condition is exacerbated by warmth and diminished with cold and is thought to result from pantothenic acid deficiency. The syndrome can be corrected with calcium pantothenate administration. PANTOTHENIC ACID DEFICIENCY • Other symptoms of deficiency include vomiting, fatigue, weakness, restlessness, and irritability. • A metabolic inhibitor of pantothenate, omega methylpantothenate, has been used in studies to induce low pantothenate status in humans. • Deficiency of pantothenic acid is thought to occur more often in conjunction with multiple nutrient deficiencies, as for example in malnutrition. PANTOTHENIC ACID DEFICIENCY • Some conditions that may increase the need for the vitamin include alcoholism, diabetes mellitus, and inflammatory bowel diseases. • Increased excretion of the vitamin has been shown in people with diabetes mellitus. Absorption is likely to be impaired with inflammatory bowel diseases. • Intake of the vitamin typically is low in people with excessive alcohol intake. PANTOTHENIC ACID TOXICITY • No toxic effects have been reported, and no UL has been established. INTERACTION WITH OTHER NUTRIENTS • In animal studies, vitamin B12, folate and biotin are required for proper use of vitamin B5 in the body’s biochemical pathways. • In addition, vitamin C appears to help prevent B5 deficiency. DIAGNOSIS OF PANTOTHENIC ACID • Blood pantothenic acid concentrations <100 mg/dL are thought to reflect low dietary pantothenate intakes; however, blood concentrations do not correlate well with changes in dietary pantothenate intake and status. • Urinary pantothenate excretion is considered to be a better indicator of status, with excretion of <1 mg/day considered indicative of poor status. REFERENCES • Gropper, S., Smith, J., & Groff, J. (2009). Advanced Nutrition and Human Metabolism. Belmont, CA: Wadsworth, Cengage Learning.