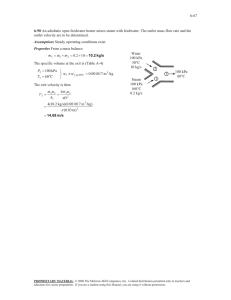
Module 2 The First Law of Thermodynamics First Law of Thermodynamics (1) Although energy assumes many forms, the total quantity of energy is constant, and when energy disappears in one form it appears simultaneously in other forms. (2) The Law of Conservation of Energy Energy can neither be created nor destroyed during a process, it can only change forms. (3) Energy Balance: Energy stored in system + Energy In = Energy stored in system + Energy Out at condition (1) at condition (2) U 1 Q KE1 PE1 system W U2 surroundings KE2 PE2 reference plane Energy Balance: U1 + KE1 + PE1 + Q + W = U2 + KE2 + PE2 Rearranging the equation: U2 – U1 + KE2 – KE1 + PE2 – PE1 = Q + W ΔU + ΔKE + ΔPE = Q + W Differential form of equation: dU + dKE + dPE = dQ + dW Application of the First Law to a Specific System: Freely Falling Body ΔU = 0 Q=0 W=0 Only potential and kinetic energies are involved: Example 1.4 (d)/p13 Energy Balance for Closed Systems ΔKE + ΔPE = 0 Closed systems often undergo processes that cause no changes in external potential energy and external kinetic energy. For such processes the energy balance reduces to: ΔU = Q + W For differential changes: dU = dQ + dW Closed system there is a flow of energy but no flow of mass across the boundary of the system. 11 | P a g e Example 2.4/p28 When a system is taken from state a to state b in Fig. 2.1 along path acb, 100 J of heat flows into the system and the system does 40 J of work. (a) How much heat flows into the system along path aeb if the work done by the system is 20 J? (b) The system returns from b to a along path bda. If the work done on the system is 30 J, does the system absorb or liberate heat? How much? Problems: 2.1, 2.5, 2.11, 2.12 and 2.30/pp 56-­‐57 Example 2.8/p39 Calculate ΔU and ΔH for 1 kg of water when it is vaporized at the constant temperature of 100oC (373.15 K) and the constant pressure of 101.325 kPa. The specific volumes of liquid and vapor water at these conditions are 0.00104 and 1.673 m3 kg-­‐1. For this change, heat in the amount of 2256.9 kJ is added to the water. Example 2.10/p43 Calculate the internal energy and enthalpy changes that occur when air is changed from an initial state of 277 K and 10 bar, where its molar volume is 2.28 m3 kmol-­‐1 to a final state of 333 K and 1 atm. Assume for air that PV/T is constant and that CV = 21 and CP = 29.3 kJ/mol-­‐1K-­‐1. Energy Balance for Isolated Systems No exchange of energy between the system and surroundings: W = 0; Q = 0 System experiences internal thermodynamic equilibrium: ΔKE = 0 ΔPE = 0 ΔU = 0 Problem 2.13/p57 A steel casting weighing 2 kg has an initial temperature of 500oC (773.15 K); 40 kg of water initially at o 25 C (298.15 K) is contained in a perfectly insulated steel tank weighing 5 kg. The casting is immersed in the water and the system is allowed to come to equilibrium. What is its final temperature? Ignore effects of expansion or contraction, and assume constant specific heats of 4.18 kJ/kg-­‐K for water and 0.50 kJ/kg-­‐K for steel. Energy Balance for Open Systems Steady Flow Energy Balance: ΔU + ΔKE + ΔPE = Q + W Work (W) consists of shaft work (WS) and net flow work (P2V2-P1V1). Substitute and rearrange; equation becomes: ΔU + Δ(PV) + ΔKE + ΔPE = Q + WS or in terms of enthalpy (OEB): ΔH + ΔKE + ΔPE = Q + WS dH + dKE + dPE = dQ + dWs 12 | P a g e Work (W) consists of: (1) shaft work (WS) – work transmitted by means of a shaft which either rotates or reciprocates. (2) flow work or energy of pressure – the entering fluid carries flow energy (P1V1) as a result of being pushed into the system (control volume) by the fluid immediately behind it. Similarly the fluid on passing point (2) does work (P2V2) by pushing the fluid just ahead of it. Net flow work done by the system = P2V2 – P1V1 The Steady-­‐State Flow Process Characteristics of a Steady flow system: (1) There is no accumulation or depletion of mass and energy within the system over the time considered (2) Thus the mass flow rate is the same at all points along the path of flow of fluid. (3) The properties (state) of the fluid may vary from point to point of the system but the properties at a given point are constant with time. Nomenclature: 𝑚 = mass flow rate (kg/s) q = volumetric flow rate (m3/s) 𝑢 = average velocity (m/s) A = cross-­‐sectional area perpendicular to direction of flow (m2) ρ = density (kg/m3) V = specific volume (m3/kg) G = mass velocity = mass flow rate per unit cross-­‐sectional area (kg/s-­‐m2) z = elevation above a datum plane Mass Balance Equations (Continuity Equations) Mass In (pt. 1) = Mass Out (pt. 2) (1) 𝑚! = 𝑚! (2) 𝑞! 𝜌! = 𝑞! 𝜌! (3) 𝑢! 𝜌! A1= 𝑢! 𝜌! A2 (4) G1A1 = G2A2 Example Problems 1. A gas flows through a horizontal 30-­‐cm inside diameter pipe at the rate of 1080 kg/min. The pipe is at an elevation of 45 m above a reference plane. The pressure of the gas is 700 kPag when the barometric pressure is 750 mmHg. The density of the gas is 0.0016 g/cm3. (a) What is the mass velocity in kg/s-­‐m2? Ans. 254.65 kg/s-­‐m2 (b) Determine the linear velocity in m/s. Ans. 159.2 m/s (c) Evaluate the different forms of energy of the gas that can possibly be calculated from given data in kJ/kg. PE = 0.441 kJ/kg KE = 12.67 kJ/kg FE (flow) = 500 kJ/kg 2. Ex 2.6-­‐1/p.51 Geankoplis. A petroleum crude oil having a density of 892 kg/m3 is flowing through the piping arrangement shown at a total rate of 1.388 x 10-­‐3 m3/s entering pipe 1.The flow divides equally in each of pipes 3. The steel pipes are schedule 40 pipe. Calculate the following SI units. (a) The total mass flow rate in pipes 1and 3. Ans. 1.238 kg/s, 0.619 kg/s (b) The average velocity in 1, 2 and 3. Ans. 0.641 m/s (pipe 1), 0.528 m/s (pipe 3) (c) The mass velocity G in 1. Ans. 572 kg/m-­‐s2 Pipe 1: 2-­‐in pipe, D1 = 2.067 in Pipe 2: 3-­‐in pipe, D2 = 3.068 in Pipe 3: 1 ½ -­‐ in pipe, D3=1.610 in 13 | P a g e 3. A fluid enters a steady-­‐flow system at a rate of 2.5 kg/s with an initial pressure of 0.70 MPa, density of 3.2 kg/m3, velocity of 30 m/s and internal energy of 1860 kJ/kg and leaves with a pressure of 0.14 MPa, density of 0.8 kg/m3, velocity of 150 m/s and internal energy of 1810 kJ/kg. During passage through the open system, each kg rejects 25 kJ of heat. Find the shaft work involve in horsepower. 1 hp = 746 W Ans. 194.2 hp Properties of Steam Definition of terms 1. Saturation temperature-­‐temperature at which liquids start to vaporize or the temperature at which vapors begin to condense. (Saturation pressure – the point where at a given temperature starts to boil) 2. Subcooled liquid-­‐one which has a temperature lower than the saturation temperature corresponding to the existing pressure. 3. Compressed liquid-­‐one which has a pressure higher the saturation pressure corresponding to the existing temperature. 4. Saturated liquid-­‐liquid at saturation temperature or saturation pressure 5. Saturated vapor-­‐vapor at the saturation conditions (about to condense) 6. Superheated vapor-­‐vapor having a temperature higher than the saturation temperature corresponding to the existing pressure. 7. Wet vapor-­‐is a combination of saturated vapor (g) and saturated liquid (f) let m = mass of wet vapor, mf = mass of the saturated liquid mg = mass of the saturated vapor m = mf + mg 8. Quality (x) of wet vapor means percent by weight that is saturated vapor 9. Percent Moisture (y) of wet vapor is the percent by weight of the of the saturated liquid Properties of wet mixture: V = Vf + x(Vg –Vf) (similarly with other properties) 4. In a steam power station, steam flows steadily through a 0.2 m diameter pipeline from the boiler to the turbine. At the boiler end, the steam is at 400oC at a pressure of 4 MPa. At the turbine entry the steam is at 392oC at a pressure of 3.5 MPa. There is a heat loss of 8.5 kJ/kg from the pipeline. Calculate the steam flow rate. Ans. 45 kg/s Some Steady-­‐Flow Engineering Devices Nozzle/Diffuser A nozzle receives a fluid and guides its expansion in an orderly manner to a lower pressure with the objective of converting some of the entering energy into kinetic energy at the exit of the nozzle. The kinetic energy is then available to drive a mechanical device. A diffuser is a device that increases the pressure of a fluid by slowing it down. 5. Steam enters an adiabatic nozzle at 1378 kPa, a velocity of 3.05 m/s, a specific volume of 0.147 m3/kg and an internal energy of 2510 kJ/kg. The exit conditions are 137.8 kPa, specific volume of 1.099 m3/kg and internal energy of 2263 kJ/kg. Determine: (a) the exit velocity of steam Ans. 772.2 m/s (b) the ratio of inlet diameter to exit diameter. Ans. 5.82 6. Air at 10oC and 80 kPa enters the diffuser of a jet engine steadily with a velocity of 200 m/s. The inlet area of the diffuser is 0.4 m2. The air leaves the diffuser with a velocity that is very small compared with the inlet velocity. If air behaves ideally, determine: (a) the mass flowrate of the air (m3/kg) (b) the enthalpy of the air leaving the diffuser (kJ/kg). H of air at 10oC = 283.14 kJ/kg 14 | P a g e Turbine In steam, gas or hydroelectric power plants, the device that drives the electric generator is the turbine. As the fluid passes through the turbine, work is done against the blades which are attached to the shaft. As a result, the shaft rotates and the turbine produces work. A turbine receives a stream of fluid at high pressure. The fluid expands to a low pressure and does work. 7. Steam is supplied to a turbine at 1.38 MPa, with an initial internal energy of 2704 kJ/kg, specific volume of 0.1665 m3/kg and a velocity of 122 m/s. Exhaust steam is at 6.9 kPa with an internal energy of 2150 kJ/kg, a specific volume of 18.316 m3/kg and a velocity of 335 m/s. The heat loss from the steam is 23 kJ/kg. Neglecting potential energy change, determine: (a) work delivered in kJ/kg Ans. –584.3 kJ/kg (b) steam flow rate in kg/hr, if the turbine delivers 100 hp Ans. 459.6 kg/hr (c) temperature of entering steam. Ans. 252.4oC 8. Steam enters and leaves a turbine through a 50 mm, med wt steel pipe. At the entrance, the steam is saturated at 2500 kPa and at the discharge, it is at 275 kPa with 90% quality. 300 kW of power are developed in the turbine but heat losses amount to 2432 kcal/hr. Determine: (a) the mass flow rate of steam used in kg/s Ans. 1.25 kg/s (b) the entering and the exit steam velocities in m/s Ans. 45.275 m/s, 335.35 m/s Compressor/Pump A compressor is capable of compressing a gas to very high pressures. In this steady-­‐flow engineering device, the work is done on the gas. Work is supplied to a compressor from an external source through a rotating shaft. Pumps work very much like compressors except that they handle liquids instead of gases. 9. Air flows steadily at the rate of 30 kg/min through an air compressor entering at a velocity of 7 m/s, a pressure of 100 kPa and a specific volume of 0.95 m3/kg. The internal energy of the air leaving compressor is 90 kJ/kg greater than that of the air entering. The air leaves at a velocity of 5 m/s, a pressure of 700 kPa and a specific volume of 0.19 m3/kg. Cooling water in the compressor jackets absorbs heat from the air at the rate of 58 kW. (a) Calculate the rate of shaft work in kW. Ans. 122 kW (b) Find the ratio of inlet pipe diameter to outlet pipe diameter. Ans. 1.89 10. A centrifugal pump operating under steady-­‐flow conditions 2.28 m3/min of water with a specific volume of 0.001 m3/kg from an initial pressure of 84 kPa to a final pressure of 280 kPa. The diameter of the suction inlet to the pump is 15 cm and the diameter of the discharge outlet is 10 cm. If the suction and discharge lines are at the same level, and no change in internal energy, determine the work (hp) done on the water by the pump. Ans. 10.46 hp 11. Example 2.16/p.54 Van Ness. Water at 200oF is pumped from a storage tank at the rate of 50 gal/min. The motor for the pump supplies work at the rate of 2 hp. The water goes through a heat exchanger, giving up heat at the rate of 40,000 BTU/min and is delivered to a second storage tank at an elevation 50 ft above the first tank. What is the temperature of the water delivered to the second tank? 100.74oF 12. Air at 1 bar and 25oC enters a compressor at low velocity and is discharged at 3 bar. The air then enters a nozzle in which it expands to a final velocity of 600m/s and returning to the initial condition of pressure and temperature. The work for compression is 240 kJ/kg of air. Determine the amount of heat to be removed during compression. Ans.: -­‐60 kJ/kg 15 | P a g e