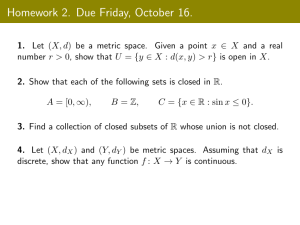
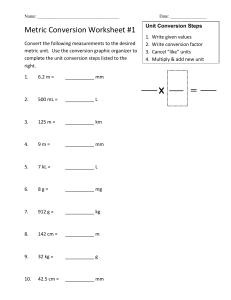
Announcements Two Youtube videos are posted and a homework Office Hours Homework- Submit in Google Classroom Competency: II -- Apply quantitative skills to represent and communicate scientific concepts. Skills: Convert between units algebraically Understand how to use the metric prefixes Questions to think about during this lesson: What is measurement? Why do we use standard units of measurement? What is the International System of Units? What are the advantages of using the SI? What are the SI units? This topic is about measurement which can be an observation or a description that includes a number and a unit. Long time ago, people measured quantities in many different ways which included using their bodies such as arms, feet and steps. These systems had many problems such as that some people do not have the same length of arm and feet. Making standard measurements proves to work well because someone at Abaarso can measure a quantity and another person in Hargeisa can also measure the same quantity in the same amount of Kilograms. Experiments must be repeatable to make accurate results and standard units allow us to repeat other scientists' experiments. The system of measurement that is best suited to scientific purpose is the metric system. The metric system was developed in France near the end of the eighteenth century and it uses the decimal basis to convert one metric to another. Because the metric system is multiples of ten, calculation can greatly be simplified by moving the decimal place. The metric system that officially scientists use is called the International System of Units which is SI Units in short. This lesson is going to distinguish between the physical quantities and units of measurement. All the things or the quantities that are measurable in the universe such as length are known as physical quantities. The units used to measure length, for instance, is meter which is the metric unit of measurement. In the table below, the seven fundamental units of measurement are shown with the seven physical quantities they measure and also their symbols for each physical quantity and SI Unit. Physical Quantity Physical Quantity SI Unit SI Unit Name Symbol Name Abbreviation Length l metre m Mass m kilogram kg Time t second s Electric Current I ampere A Temperature T Kelvin K Amount of Substance n mole mol Luminous Intensity l candela cd Physical quantity Physical quantity SI Unit SI unit Name Symbol Abbreviation Name m2 area A volume V m3 cubic meter frequency f Hz Hertz velocity v acceleration a m s –1 m s –2 square meter meters per second meters per second squared force F N Newton pressure P or p Pa Pascal power P W Watt energy E J Joule voltage V V Volt resistance R Ω Ohm conductance G S Siemens charge Q C Coulomb