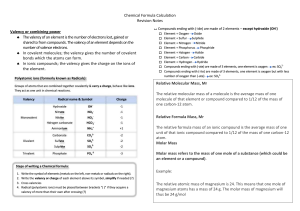
Topic 1 recap questions Define the followings; 1. Empirical formula: The simplest mole ratio of atoms in a compound 2. Molecular formula: The actual number of atoms in a compound 3. Mixture: two or more types of atoms joined together not chemically 4. Element: only type of atoms 5. Atom: The smallest stable part of an element (basic unit) 6. Compound: two or more types of atoms joined together chemically 7. Avogadro’s constant: The number of particles in 1 mole of any substance, 6.02x1023 8. Relative atomic mass (Ar): Average mass of an atom of an element compared to 1/12 mass of an atom of carbon-12 9. Relative molecular mass (Mr): Average mass of a compound or molecule compared to 1/12 mass of an atom of carbon-12 10. Isotopes: Atoms with same number of protons but different number of neutrons therefore different mass number ` 1. H2 + Cu2O -> 2Cu + H2O 2. C + CO2 -> 2CO 3. Mg + H2SO4 -> H2 + MgSO4 4. Cu + Cl2 -> CuCl2 5. 2Hg+ O2 -> 2HgO 6. Fe + S -> FeS 0.020 x (40.08+32.07+16x4) = 2.723 g ----(x1000)---- 2723 mg 10 / (47.87+35.45x4) x (47.87 + 16x2) = 4.21g • Q8, 14, 20 Zn 40.6/65.38 /smallest number 0.61/0.6 Ratio 1 Formula ZnSO4 S 19.8/32.07 0.61/0.6 : 1 O 39.6/16 2.4/0.4 :4