Particulate Nature of Matter: Solids, Liquids, Gases Model
advertisement

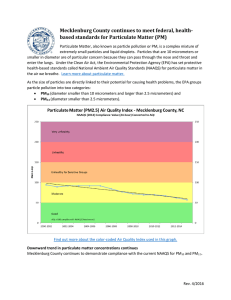
Chapter 7Model of Matter — The Particulate Nature of Matter Learning Outcomes • show an awareness that according to the Particulate Nature of Matter, matter is made up of small discrete particles which are in constant and random motion • show an understanding of the simple model of solids, liquids and gases, in terms of arrangement and movement of particles • use models to explain melting and boiling in terms of conversion of the three states of matter THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Learning Outcomes • use models to explain expansion and contraction, and the conservation of mass during these processes • compare the properties of solids, liquids and gases in terms of arrangement and movement of particles • show an appreciation of how in practice, models are constructed to explain phenomena • show an appreciation of scientific attitudes such as creativity and open-mindedness in creating models to explain the fundamental nature of things and the willingness to re-examine existing models THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Part I THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter , s d li o s g n o m a e r the e r a s e c n e r fe if d t Wha ? s e s a g d n a s id u q li tter a m f o l e d o m e t la Why is the particu important? THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter What is matter made of? THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter What is the science behind making popsicles? THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter 7.1 What Is Matter Made Of? • The particulate theory of matter describes what matter is made of. • Scientists believe that matter is made up of very small particles. A particle is a word we use for a small piece of matter. • We can see small pieces of sugar. However, scientists believe that sugar is made up of even smaller particles that we cannot see. THEME B: MODELS Chapter THEME B: 7Model of Matter — The Particulate Nature of Matter MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Evidence for spaces between particles • If 30 cm3 of water is mixed with 30 cm3 of alcohol, the total volume is less than 60 cm3. Why is this so? THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Evidence for spaces between particles • If we mix 30 cm3 of rice and 30 cm3 of beans, the total volume is also less than 60 cm3. • This is because there are spaces between the rice grains and the beans. On mixing, the rice grains move into the spaces between the beans. • This suggests that water and alcohol are also made of particles and that there are spaces between the particles. THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter In step 2 of making popsicles, we add sugar to water and obtain a sugar solution as a result. Where do the sugar particles ‘disappear’ to? THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Evidence for different spacing of particles in solids, liquids and gases • Gases are easier to compress than liquids or solids. • Click on the arrows to compress the balloon and the rock. THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Evidence for different spacing of particles in solids, liquids and gases • The particles in a gas are far apart. When we squeeze a balloon, we are actually pushing the particles closer together. • The spaces between the particles of a solid are too small. We cannot push the particles closer to one another and hence we cannot compress solids. THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Evidence for movement of particles • If a bottle of perfume is opened in a corner of a room, the smell of perfume spreads to all parts of the room. This suggests that small invisible perfume particles are moving. • The movement of the particles of matter is described as random, that is, they move in all directions and not in a specific one. THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter 1. What do scientists believe matter is made up of? 2. There are spaces between particles of matter. How do the spaces differ in solids, liquids and gases? 3. Give some everyday examples to show that particles are in motion. THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter 7.2 How Can We Understand the Particulate Nature of Matter? • In science, we make models to understand things we cannot observe directly. • As particles of matter are too small to be seen, scientists have constructed a physical model to show how particles in the solid, liquid and gaseous states of a substance are arranged. It is called the particulate model of matter and helps us to explain the particulate nature of matter. • This model also helps us to visualise what solids, liquids and gases are like on the inside. THEME B: MODELS Chapter THEME B: 7Model of Matter — The Particulate Nature of Matter MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Using the particulate model of matter to explain properties of matter THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Solids THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Liquids THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Gases THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Using the particulate model of matter to explain expansion and contraction • Substances expand when heated and contract when cooled. • When cooled, particles lose energy, move slower and closer to one another. Thus, the volume decreases. • When heated, particles gain energy, move faster and further away from one another. Thus, the volume increases. THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Using the particulate model of matter to explain expansion and contraction • During expansion and contraction, only the distances between the particles change. • The size and mass of the particles do not change. • We can use the particulate model of matter to predict that the mass of the substance remains the same, although the volume of the substance changes when it expands or contracts. THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Some water is heated in a saucepan. What happens to the motion and spaces between water particles when heat is applied? How does this affect step 2 of making the popsicle? THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Journal Entry 1. Use the particulate model of matter to explain the following. (a) A liquid is diffcult to compress. (b) If we squeeze a solid, its shape and size do not change. (c) If we squeeze a balloon, its shape can change. (d) A hot gas is less dense than the same gas when it is cold. (e) A liquid can flow and take the shape of its container. THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Journal Entry 3. In which state of matter are the particles (a) closest together? (b) moving the fastest? (c) able to move short distances only? THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Part II THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter 7.3 Using the Particulate Model to Explain Changes of State [Optional for N(A)] • The particulate model of matter can be used to explain changes of state. • During a change in state, the temperature does not change. The energy added during the change of state is used to move particles further apart. • When water boils, the temperature remains at 100 °C until all the water has changed into steam. • The energy added during this change is used by the particles to increase the distance between them. THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Changes of state in matter Click the arrows to view the process. http://www.dlt.ncssm.edu/TIGER/F lash/phase/HeatingCurve.html THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Heating Curve Liquid boiling Solid melting What do THEME B: MODELS and represent? Why does the temperature remain the same? Chapter 7Model of Matter — The Particulate Nature of Matter Heating Process THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter What happen when heat is applied to liquid • Particle will gain kinetic energy • Moves faster as temperature rises Boiling Particles have energy to overcome the forces(of attraction) that hold them together Liquid become gas Temperature where it occurs is boiling point THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter In step 4, the lemon mixture is poured into a mould which is then placed in the freezer. What will happen to the distance between the particles of the mixture as it cools? THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter 1. In making a solid metal object, a liquid metal is poured into a container called a mould. The mould gives shape to the metal when it cools and hardens. How does metal casting make use of the different characteristics of liquids and solids? 2. Solid X turns into a liquid at 80 °C and into a gas at 140 °C. Describe the changes in the particles of X when it is heated from (a) 70 °C to 85 °C, THEME B: MODELS (b) 85 °C to 145 °C. Chapter 7Model of Matter — The Particulate Nature of Matter Chapter Review THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Chapter Review THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Chapter Review THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Chapter Review THEME B: MODELS Chapter 7Model of Matter — The Particulate Nature of Matter Chapter Review THEME B: MODELS