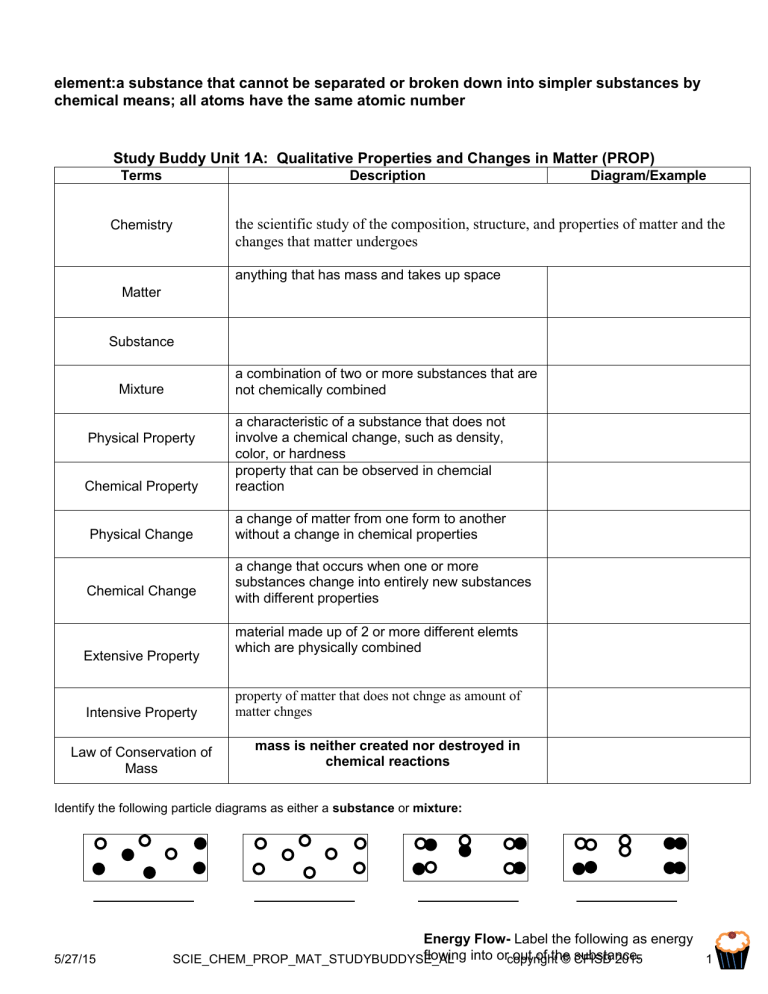
element:a substance that cannot be separated or broken down into simpler substances by chemical means; all atoms have the same atomic number Study Buddy Unit 1A: Qualitative Properties and Changes in Matter (PROP) Terms Description Diagram/Example the scientific study of the composition, structure, and properties of matter and the changes that matter undergoes Chemistry anything that has mass and takes up space Matter Substance a combination of two or more substances that are not chemically combined Mixture Chemical Property a characteristic of a substance that does not involve a chemical change, such as density, color, or hardness property that can be observed in chemcial reaction Physical Change a change of matter from one form to another without a change in chemical properties Physical Property Chemical Change Extensive Property Intensive Property Law of Conservation of Mass a change that occurs when one or more substances change into entirely new substances with different properties material made up of 2 or more different elemts which are physically combined property of matter that does not chnge as amount of matter chnges mass is neither created nor destroyed in chemical reactions Identify the following particle diagrams as either a substance or mixture: 5/27/15 Energy Flow- Label the following as energy flowing into orcopyright out of the substance. SCIE_CHEM_PROP_MAT_STUDYBUDDYSE_AL © CFISD 2015 1 Physical & Chemical Practice Density Color Boiling Point Evaporation Burning Property or Change Physical or Chemical Intensive or Extensive property property property change change physical physical chemical chemical physical intensive intensive intensive Example Baking a cupcake Freezing water Fireworks exploding Where is the energy going? inside outside inside Study Buddy Unit 1B: Quantitative Measurement of Matter (PROP) Terms Description a measure of the amount of matter that an object contains; the SI base unit of mass is the kilogram Mass Symbol Unit a measure of the space occupied by a sample of matter the ratio of the mass of an object to its volume Volume Density m/v the amount of a substance that contains 6.02 × 1023 representative particles of that substance Mole Avogadro’s Number Molar Mass the number of representative particles contained in one mole of a substance; equal to 6.02 × 1023 particles the mass of one mole of any substance- look at PT atomic mass for elements Accuracy:the closeness of a measurement to the true value of what is being measured Precision: describes the closeness, or reproducibility, of a set of measurements taken under the same conditions Rank the States of Matter (Solid, Liquid, Gas) from Most to Least Dense for Most Substances Most dense _____, _____, _____ Least Dense Using the image above, what is the length of this nail to: 1 significant figure 2 significant figures 5/27/15 SCIE_CHEM_PROP_MAT_STUDYBUDDYSE_AL copyright © CFISD 2015 2 3 significant figures Circle the measurement that is most accurate ↑ Convert the following numbers between Standard and Scientific Notation Standard Notation 0.0026 6,000,000 0.000092 620000 .000198 34000000 Scientific Notation 2.6x10*-3 6x10*7 9.2x10*5 6.2 x 105 1.98 x 10-4 3.4 x 107 Round the molar mass of the following elements to the tenths place: Molar Mass (g/mol) Find atomic mass and then find the how many moles and then multiply for atoms you do 6.02X10*23 and times taht by moles Oxygen Chlorine Hydrogen Iron Calcium Place Value: 1000 1 (g, L, m) 0.01 0.001 SI Prefix: 1 mole of Carbon = _____________ atoms Fraction: = _____________ grams Convert 1503.5 centimeters to kilometers. = 5/27/15 = or SCIE_CHEM_PROP_MAT_STUDYBUDDYSE_AL X X copyright © CFISD 2015 = 3

